|
|
|
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Adults are resistant to the bacterium that causes Botulism. These bacteria thrive in honey – therefore, honey should never be given to infants since their immune systems are not yet resistant.
Persons who overdose with cardiac glycosides have a better chance of overall survival if they can survive the first 24 hours after the overdose.
Many people have small pouches in their colons that bulge outward through weak spots. Each pouch is called a diverticulum. About 10% of Americans older than age 40 years have diverticulosis, which, when the pouches become infected or inflamed, is called diverticulitis. The main cause of diverticular disease is a low-fiber diet.
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
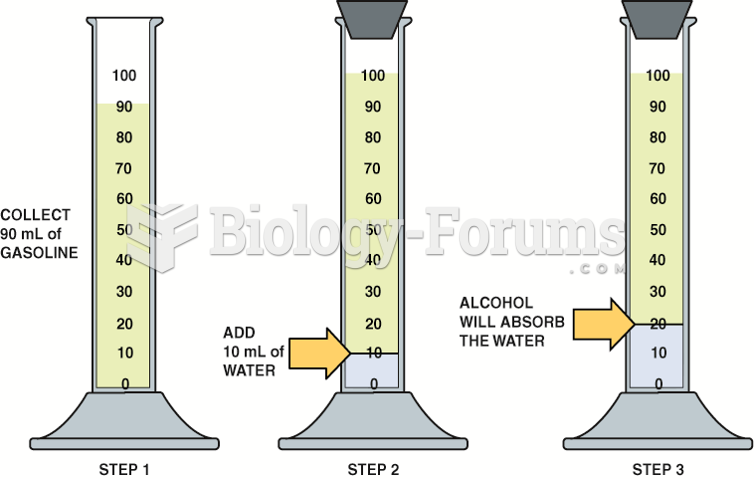 Checking gasoline for alcohol involves using a graduated cylinder and adding water to check if the ...
Checking gasoline for alcohol involves using a graduated cylinder and adding water to check if the ...
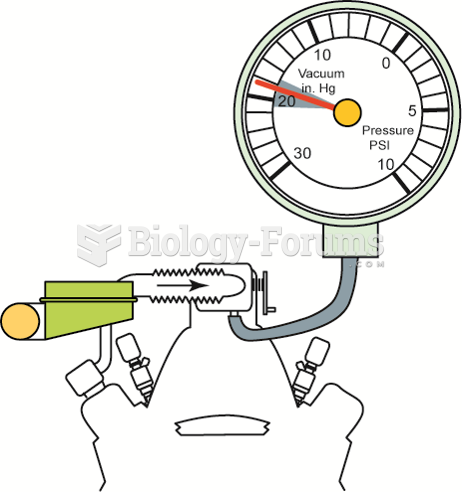 An engine in good mechanical condition should produce 17 to 21 inches Hg of vacuum at idle at sea ...
An engine in good mechanical condition should produce 17 to 21 inches Hg of vacuum at idle at sea ...