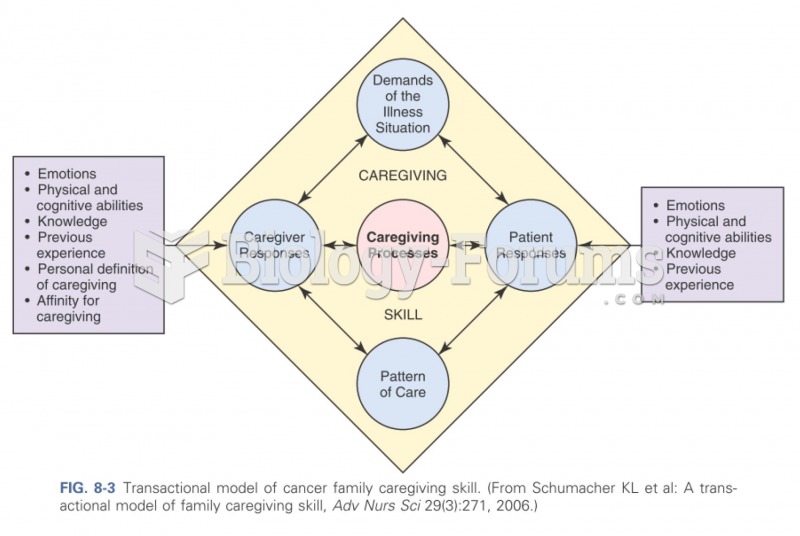
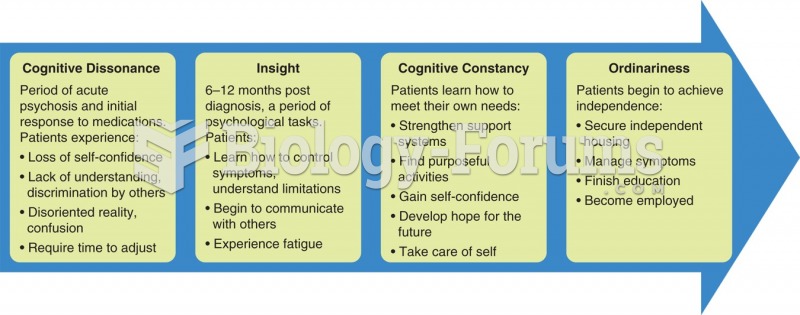
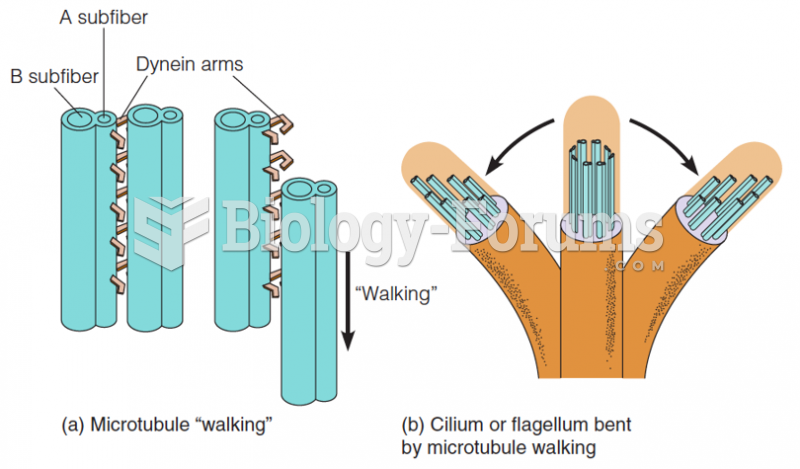
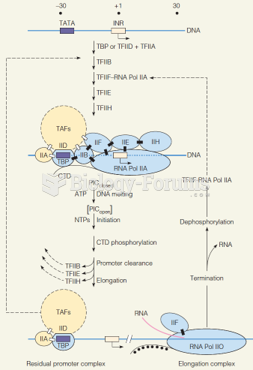
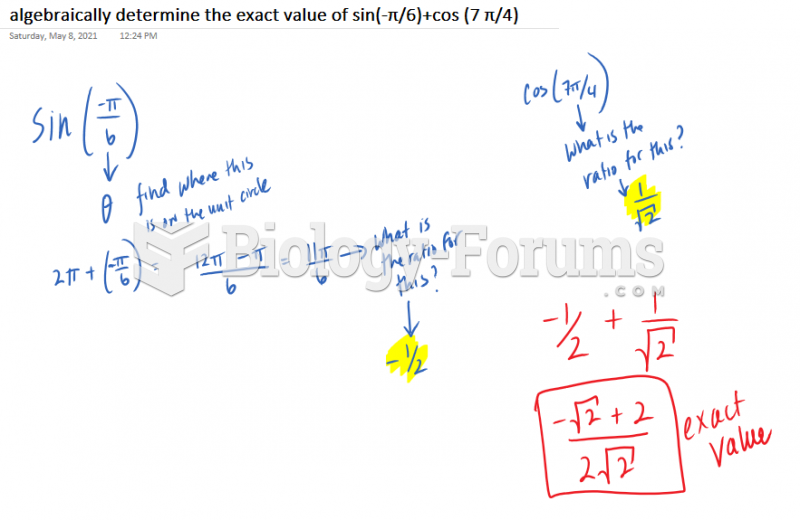
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
According to research, pregnant women tend to eat more if carrying a baby boy. Male fetuses may secrete a chemical that stimulates their mothers to step up her energy intake.
Did you know?
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
Did you know?
Alzheimer's disease affects only about 10% of people older than 65 years of age. Most forms of decreased mental function and dementia are caused by disuse (letting the mind get lazy).