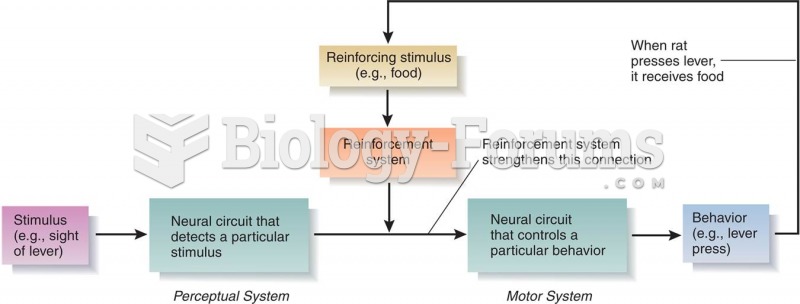
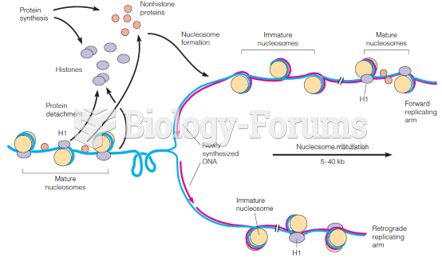
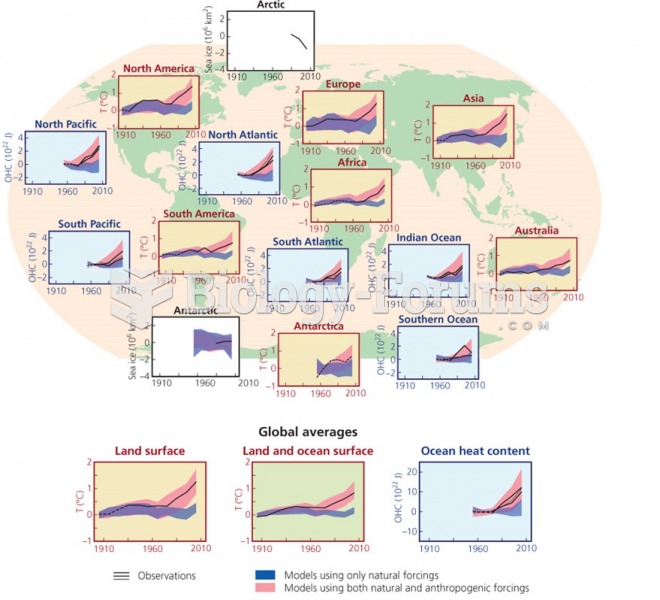
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.
Did you know?
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
Did you know?
In the United States, congenital cytomegalovirus causes one child to become disabled almost every hour. CMV is the leading preventable viral cause of development disability in newborns. These disabilities include hearing or vision loss, and cerebral palsy.
Did you know?
Women are 50% to 75% more likely than men to experience an adverse drug reaction.