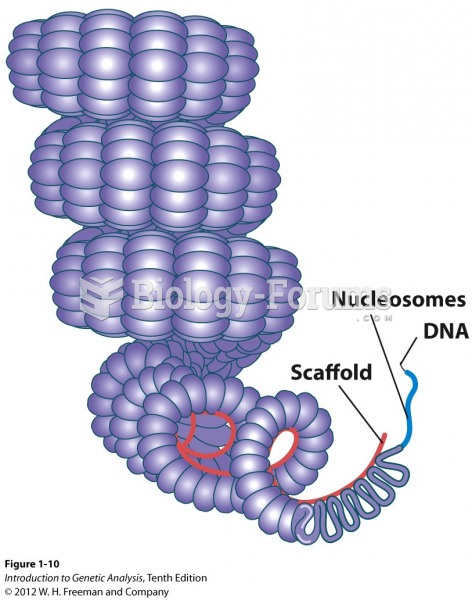
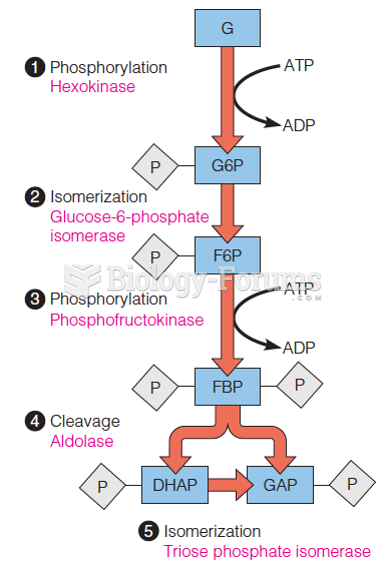
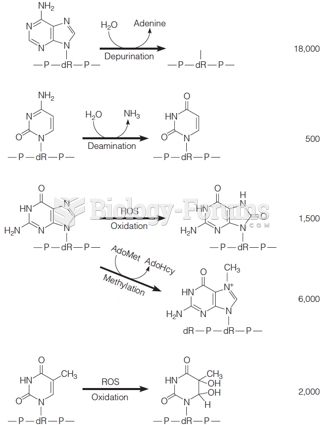
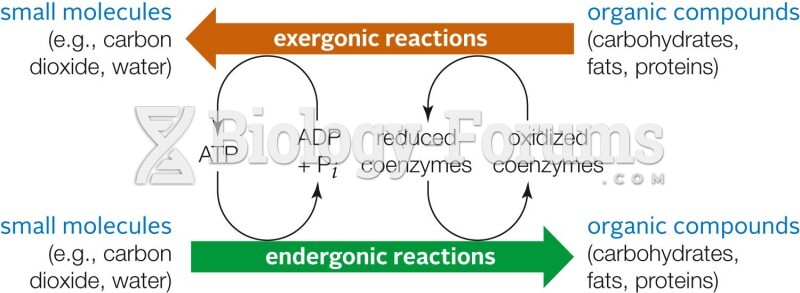
|
|
|
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
There are approximately 3 million unintended pregnancies in the United States each year.
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
A strange skin disease referred to as Morgellons has occurred in the southern United States and in California. Symptoms include slowly healing sores, joint pain, persistent fatigue, and a sensation of things crawling through the skin. Another symptom is strange-looking, threadlike extrusions coming out of the skin.
The modern decimal position system was the invention of the Hindus (around 800 AD), involving the placing of numerals to indicate their value (units, tens, hundreds, and so on).