|
|
|
Prostaglandins were first isolated from human semen in Sweden in the 1930s. They were so named because the researcher thought that they came from the prostate gland. In fact, prostaglandins exist and are synthesized in almost every cell of the body.
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.
In 1885, the Lloyd Manufacturing Company of Albany, New York, promoted and sold "Cocaine Toothache Drops" at 15 cents per bottle! In 1914, the Harrison Narcotic Act brought the sale and distribution of this drug under federal control.
 Field research on free-living primates allows primatologists to study patterns of behavior in the se
Field research on free-living primates allows primatologists to study patterns of behavior in the se
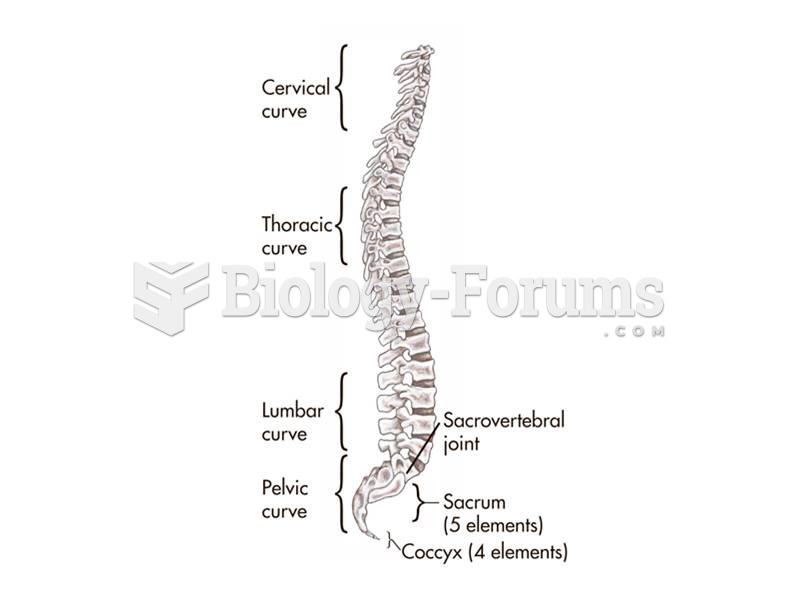 The S-curve in the human vertebral column--a result of the evolution of bipedality--makes humans hig
The S-curve in the human vertebral column--a result of the evolution of bipedality--makes humans hig