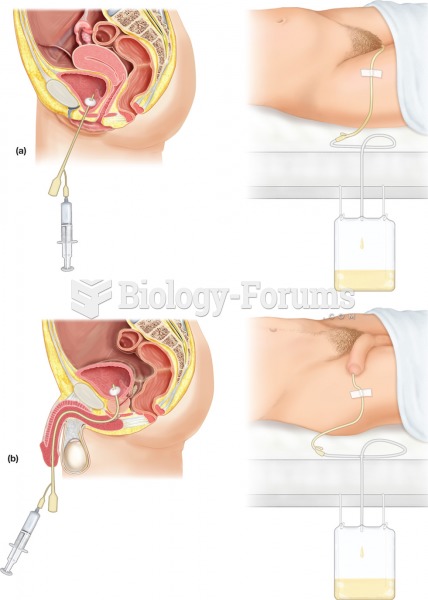
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The effects of organophosphate poisoning are referred to by using the abbreviations “SLUD” or “SLUDGE,” It stands for: salivation, lacrimation, urination, defecation, GI upset, and emesis.
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
Did you know?
There are over 65,000 known species of protozoa. About 10,000 species are parasitic.
Did you know?
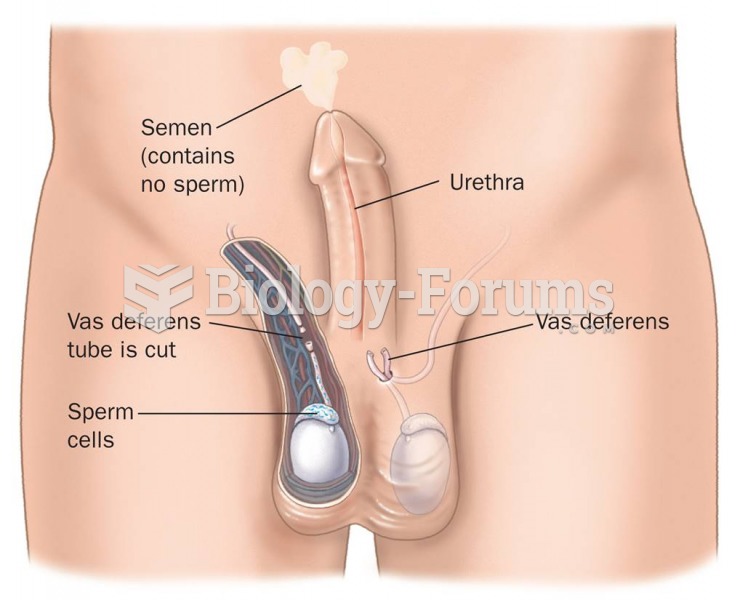
Sperm cells are so tiny that 400 to 500 million (400,000,000–500,000,000) of them fit onto 1 tsp.
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.