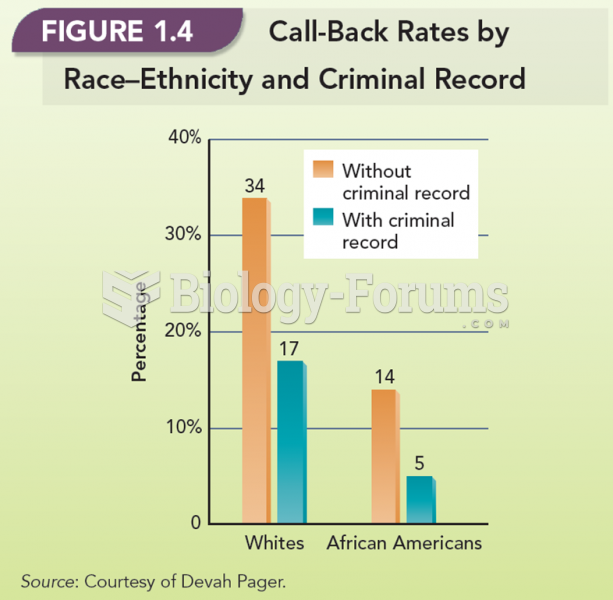
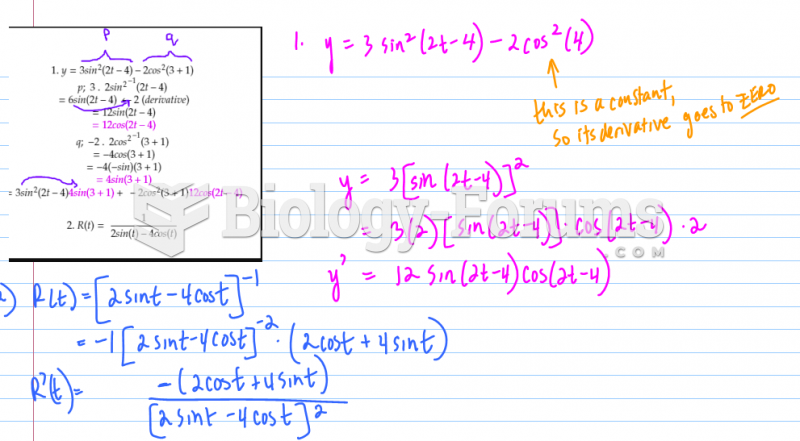
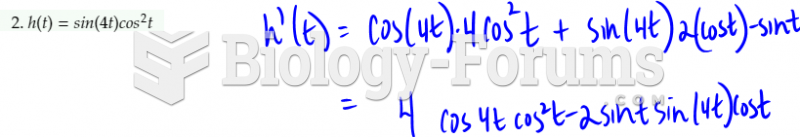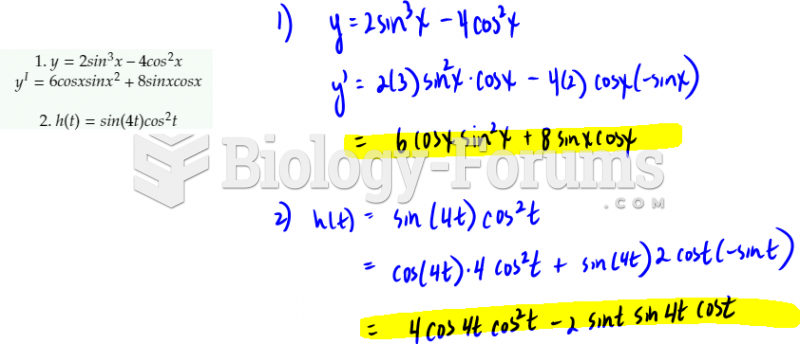The following diol is prepared from propanal (CH3CH2CHO) and 3-bromopropanol. Which derivatives will be among intermediate products in this transformation?

1.a trimethylsilyl ether and a Grignard reagent
2.an enol and a ketone
3.a Grignard reagent and a ketone
4.an alkoxide and an ester
5.an organocopper reagent and an alkyne
Question 2Phenol is treated with Fremys salt, (KSO3)2NO, followed by SnCl2/H2O. What is the product of these manipulations?
 Question 3
Question 3Which of the following would not be a good method to prepare 2-butanone?
1.2-butanol with Dess-Martin periodinane
2.methyl acetate with methyl Grignard
3.2-butyne with borane followed by basic hydrogen peroxide
4.2-butyne with HgSO4 in H2SO4/H2O
5.3,4-dimethylhex-3-ene with KMnO4/H+
Question 4Which set of reagents is the best to perform the following transformation?

1.1. Dess-Martin periodinane, 2. CH3MgBr, 3. POCl3/pyridine
2.1. tosyl chloride, 2. CH3MgBr
3.1. HBr, 2. Mgo, 3. CH3I
4.1. NaOH, 2. CH3I
5.1. CrO3/H3O+, 2. NaBH4, 3. CH3MgBr
Question 5What is the major elimination product in the following reaction?

1.3-methyl-3-hexene
2.3-methyl-1-hexene
3.3-methyl-2-hexene
4.2-ethyl-1-pentene
5.2-ethyl-2-pentene
Question 6Which is the best hydride reagent to carry out the following reaction?

1.NaBH4
2.NaH
3.BH3
4.LiAlH4
5.H2
Question 7What is the product of the following reaction?
 Question 8
Question 8What is the product of the above sequence of reactions?

 Question 9
Question 9What is the correct order of acidity, starting with the least acidic, for the following phenols?

1.A < B < C < D
2.D < C < A < B
3.C < D < B < A
4.C < D < A < B
5.D < C < B < A
Question 10Chiral alcohols can be converted to chlorides by several pathways. Which is the best way to make the chloride with retention of configuration?
1.reaction with HCl
2.reaction with PBr3 followed by NaCl
3.reaction with tosyl chloride, followed by hydroxide
4.reaction with KI followed by NaCl