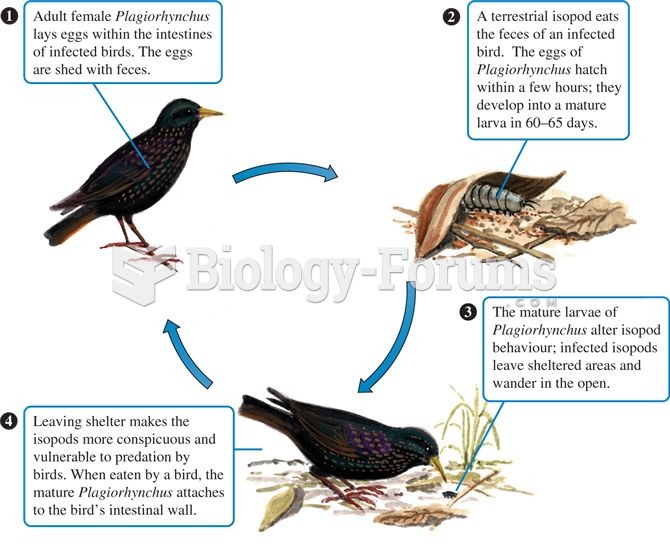
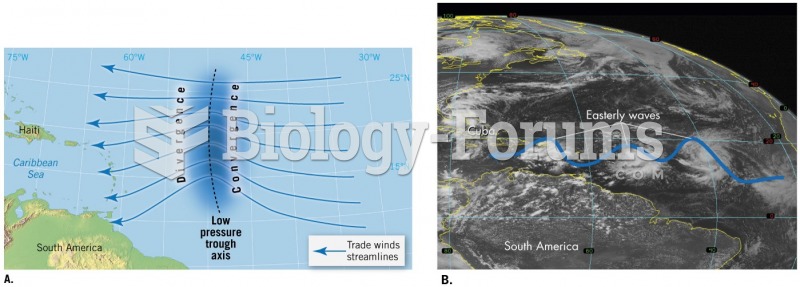
|
|
|
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.
A cataract is a clouding of the eyes' natural lens. As we age, some clouding of the lens may occur. The first sign of a cataract is usually blurry vision. Although glasses and other visual aids may at first help a person with cataracts, surgery may become inevitable. Cataract surgery is very successful in restoring vision, and it is the most frequently performed surgery in the United States.
Eating food that has been cooked with poppy seeds may cause you to fail a drug screening test, because the seeds contain enough opiate alkaloids to register as a positive.
The largest baby ever born weighed more than 23 pounds but died just 11 hours after his birth in 1879. The largest surviving baby was born in October 2009 in Sumatra, Indonesia, and weighed an astounding 19.2 pounds at birth.
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
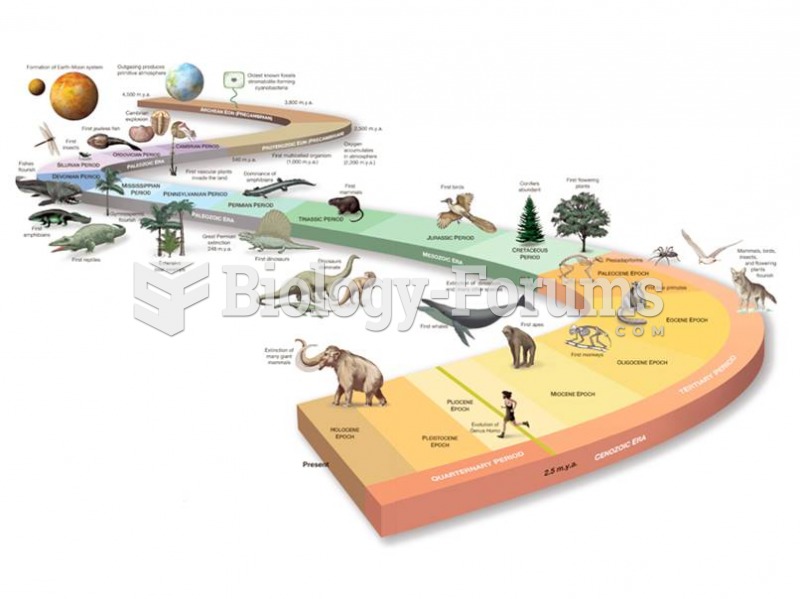 Earth’s history spans 4.5 billion years. Geologists and paleontologists have pieced together the his
Earth’s history spans 4.5 billion years. Geologists and paleontologists have pieced together the his
 This cover of Life (1925) offered a stereotypical rendering of the new generation: a young couple ..
This cover of Life (1925) offered a stereotypical rendering of the new generation: a young couple ..