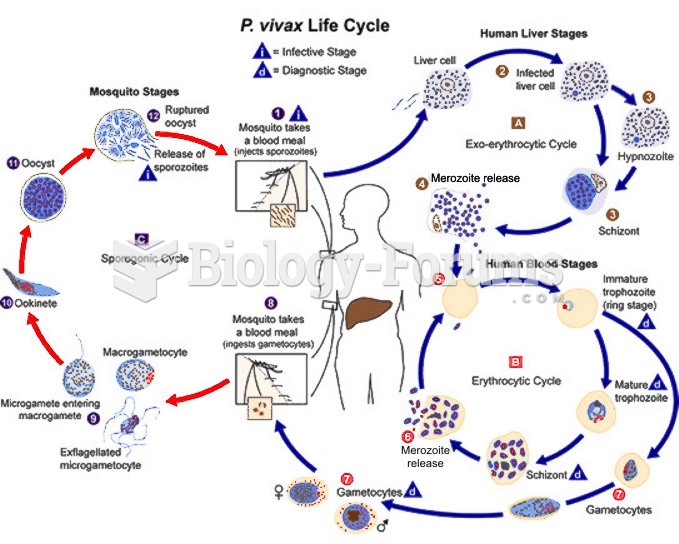
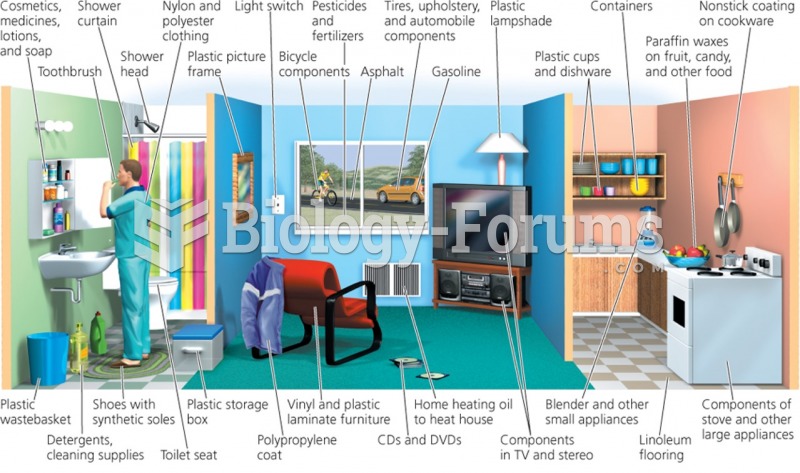
|
|
|
Asthma cases in Americans are about 75% higher today than they were in 1980.
An identified risk factor for osteoporosis is the intake of excessive amounts of vitamin A. Dietary intake of approximately double the recommended daily amount of vitamin A, by women, has been shown to reduce bone mineral density and increase the chances for hip fractures compared with women who consumed the recommended daily amount (or less) of vitamin A.
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.
Your heart beats over 36 million times a year.
A seasonal flu vaccine is the best way to reduce the chances you will get seasonal influenza and spread it to others.