This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Asthma is the most common chronic childhood disease in the world. Most children who develop asthma have symptoms before they are 5 years old.
Did you know?
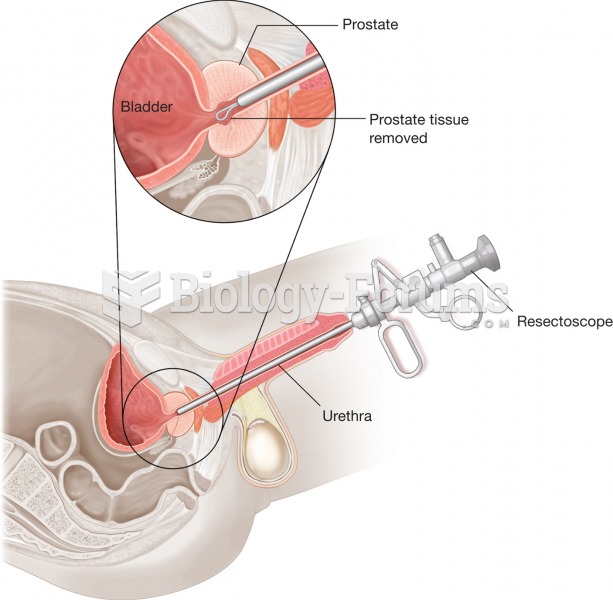
In most cases, kidneys can recover from almost complete loss of function, such as in acute kidney (renal) failure.
Did you know?
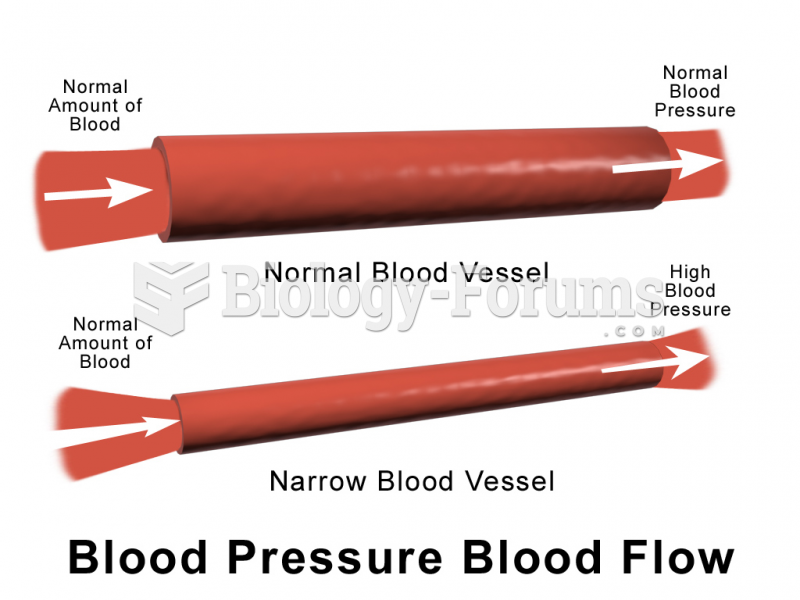
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
Did you know?
Barbituric acid, the base material of barbiturates, was first synthesized in 1863 by Adolph von Bayer. His company later went on to synthesize aspirin for the first time, and Bayer aspirin is still a popular brand today.
Did you know?
There are approximately 3 million unintended pregnancies in the United States each year.