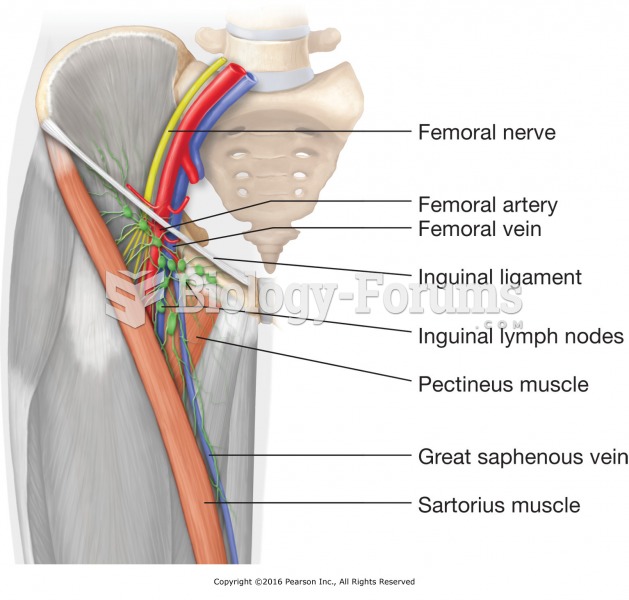
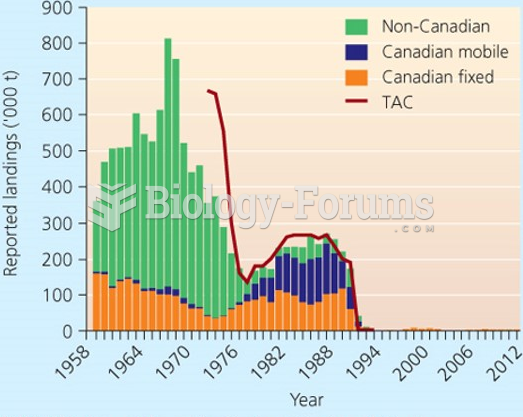
|
|
|
There are major differences in the metabolism of morphine and the illegal drug heroin. Morphine mostly produces its CNS effects through m-receptors, and at k- and d-receptors. Heroin has a slight affinity for opiate receptors. Most of its actions are due to metabolism to active metabolites (6-acetylmorphine, morphine, and morphine-6-glucuronide).
The use of salicylates dates back 2,500 years to Hippocrates’s recommendation of willow bark (from which a salicylate is derived) as an aid to the pains of childbirth. However, overdosage of salicylates can harm body fluids, electrolytes, the CNS, the GI tract, the ears, the lungs, the blood, the liver, and the kidneys and cause coma or death.
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
Always store hazardous household chemicals in their original containers out of reach of children. These include bleach, paint, strippers and products containing turpentine, garden chemicals, oven cleaners, fondue fuels, nail polish, and nail polish remover.
The average adult has about 21 square feet of skin.
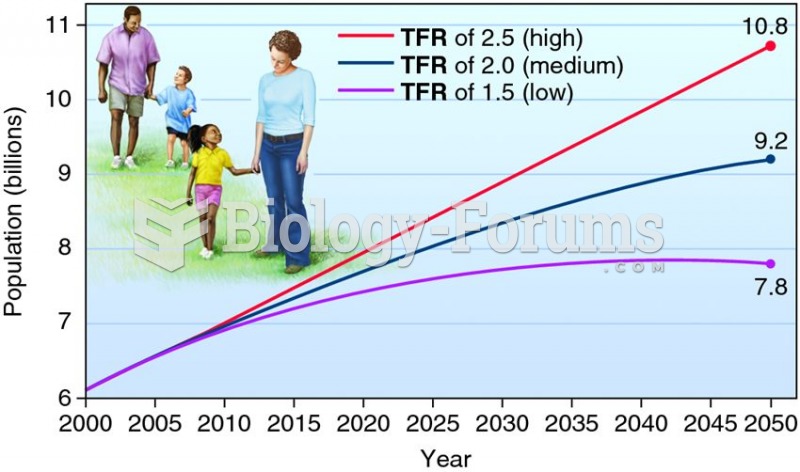 Population predictions for 2000–2050, using three different total fertility rates (
Population predictions for 2000–2050, using three different total fertility rates (
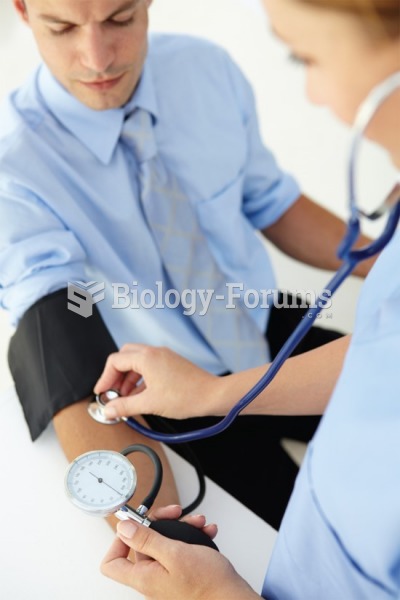 Taking a blood pressure is one of the procedures the medical assistant extern may perform under the ...
Taking a blood pressure is one of the procedures the medical assistant extern may perform under the ...