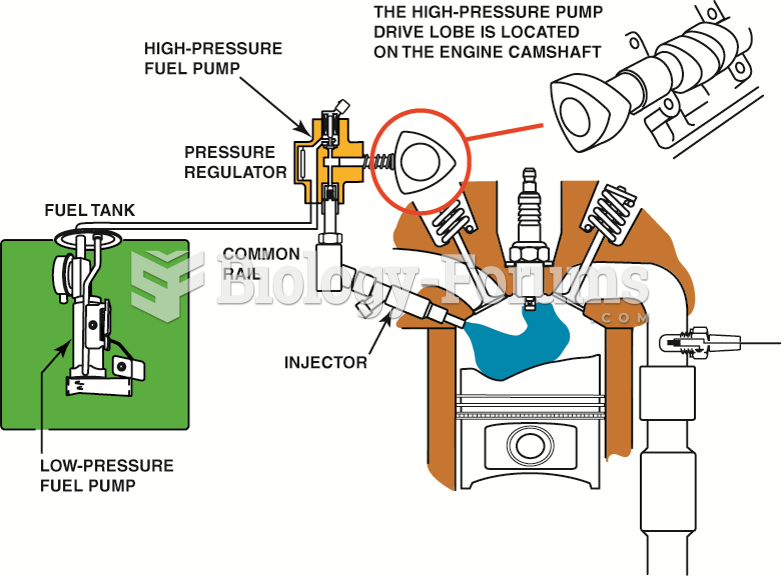
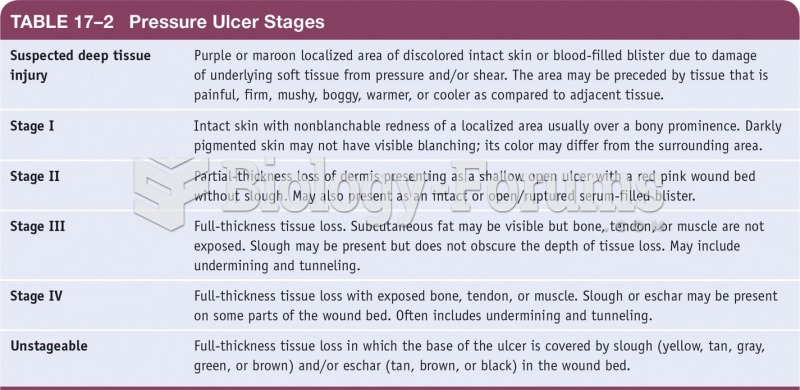
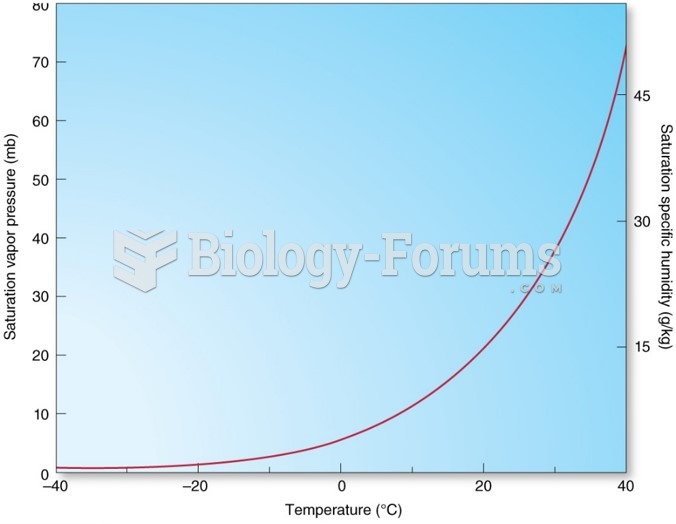
|
|
|
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.
As many as 20% of Americans have been infected by the fungus known as Histoplasmosis. While most people are asymptomatic or only have slight symptoms, infection can progress to a rapid and potentially fatal superinfection.
If you use artificial sweeteners, such as cyclamates, your eyes may be more sensitive to light. Other factors that will make your eyes more sensitive to light include use of antibiotics, oral contraceptives, hypertension medications, diuretics, and antidiabetic medications.
The highest suicide rate in the United States is among people ages 65 years and older. Almost 15% of people in this age group commit suicide every year.
There are actually 60 minerals, 16 vitamins, 12 essential amino acids, and three essential fatty acids that your body needs every day.
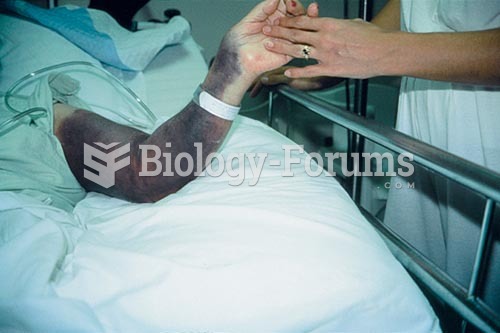 Proper placement and monitoring of an automatic blood pressure cuff will reduce the risk of injury o
Proper placement and monitoring of an automatic blood pressure cuff will reduce the risk of injury o
 Sphygmomanometry. Photograph of a physician taking blood pressure readings with the use of a sphygmo
Sphygmomanometry. Photograph of a physician taking blood pressure readings with the use of a sphygmo