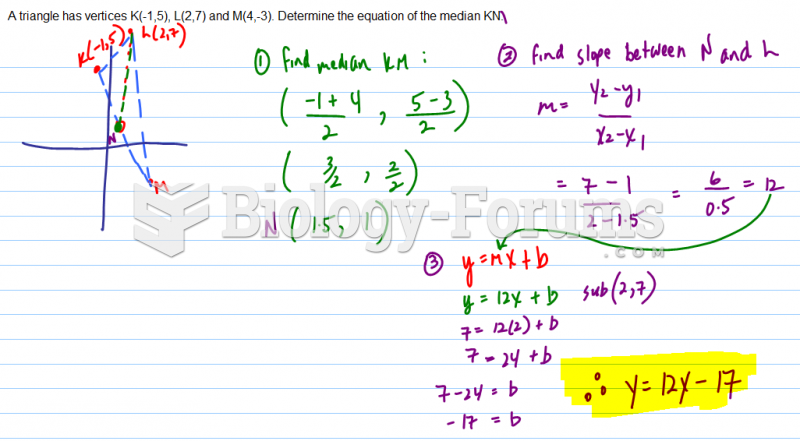
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
After a vasectomy, it takes about 12 ejaculations to clear out sperm that were already beyond the blocked area.
Did you know?
Signs of depression include feeling sad most of the time for 2 weeks or longer; loss of interest in things normally enjoyed; lack of energy; sleep and appetite disturbances; weight changes; feelings of hopelessness, helplessness, or worthlessness; an inability to make decisions; and thoughts of death and suicide.
Did you know?
Nearly 31 million adults in America have a total cholesterol level that is more than 240 mg per dL.
Did you know?
The word drug comes from the Dutch word droog (meaning "dry"). For centuries, most drugs came from dried plants, hence the name.
Did you know?
The FDA recognizes 118 routes of administration.