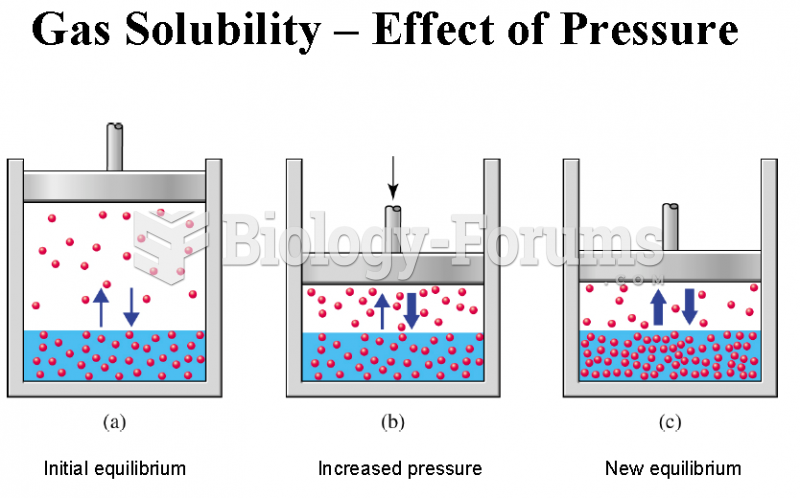
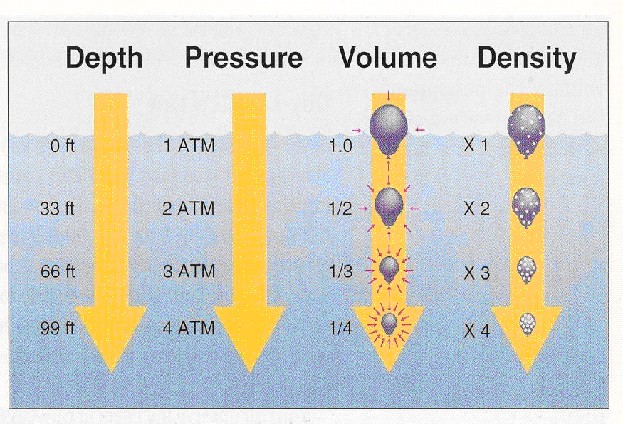
|
|
|
An identified risk factor for osteoporosis is the intake of excessive amounts of vitamin A. Dietary intake of approximately double the recommended daily amount of vitamin A, by women, has been shown to reduce bone mineral density and increase the chances for hip fractures compared with women who consumed the recommended daily amount (or less) of vitamin A.
People with high total cholesterol have about two times the risk for heart disease as people with ideal levels.
In ancient Rome, many of the richer people in the population had lead-induced gout. The reason for this is unclear. Lead poisoning has also been linked to madness.
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Women are 50% to 75% more likely than men to experience an adverse drug reaction.