This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
People about to have surgery must tell their health care providers about all supplements they take.
Did you know?
Excessive alcohol use costs the country approximately $235 billion every year.
Did you know?
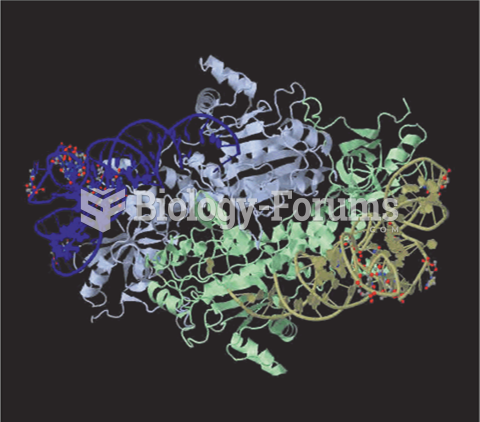
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Did you know?
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Did you know?
Cucumber slices relieve headaches by tightening blood vessels, reducing blood flow to the area, and relieving pressure.