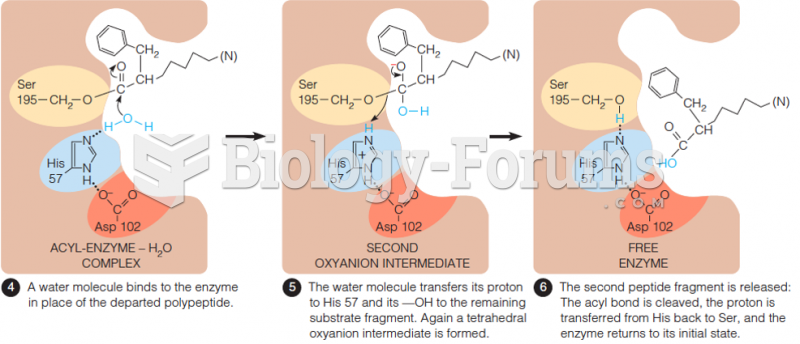
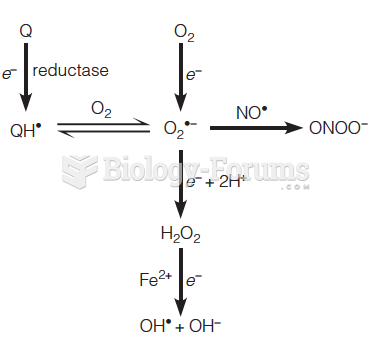
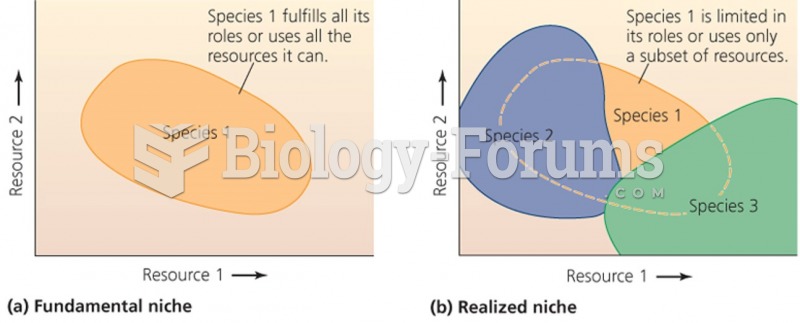
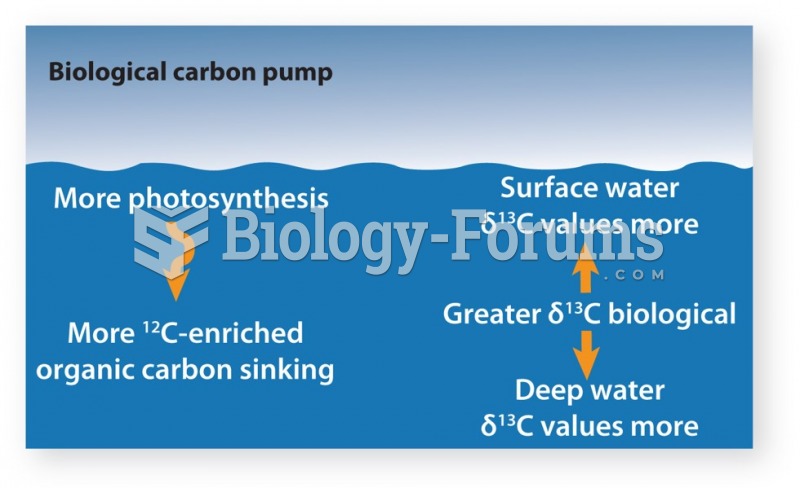
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The first-known contraceptive was crocodile dung, used in Egypt in 2000 BC. Condoms were also reportedly used, made of animal bladders or intestines.
Did you know?
Approximately 25% of all reported medication errors result from some kind of name confusion.
Did you know?
The human body produces and destroys 15 million blood cells every second.
Did you know?
It is widely believed that giving a daily oral dose of aspirin to heart attack patients improves their chances of survival because the aspirin blocks the formation of new blood clots.
Did you know?
The modern decimal position system was the invention of the Hindus (around 800 AD), involving the placing of numerals to indicate their value (units, tens, hundreds, and so on).