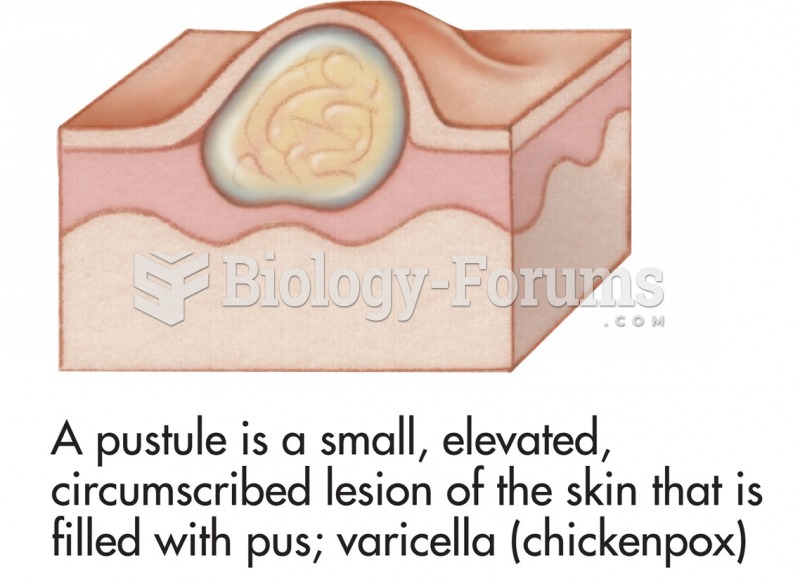
|
|
|
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.
The liver is the only organ that has the ability to regenerate itself after certain types of damage. As much as 25% of the liver can be removed, and it will still regenerate back to its original shape and size. However, the liver cannot regenerate after severe damage caused by alcohol.
Common abbreviations that cause medication errors include U (unit), mg (milligram), QD (every day), SC (subcutaneous), TIW (three times per week), D/C (discharge or discontinue), HS (at bedtime or "hours of sleep"), cc (cubic centimeters), and AU (each ear).
Excessive alcohol use costs the country approximately $235 billion every year.
It is difficult to obtain enough calcium without consuming milk or other dairy foods.