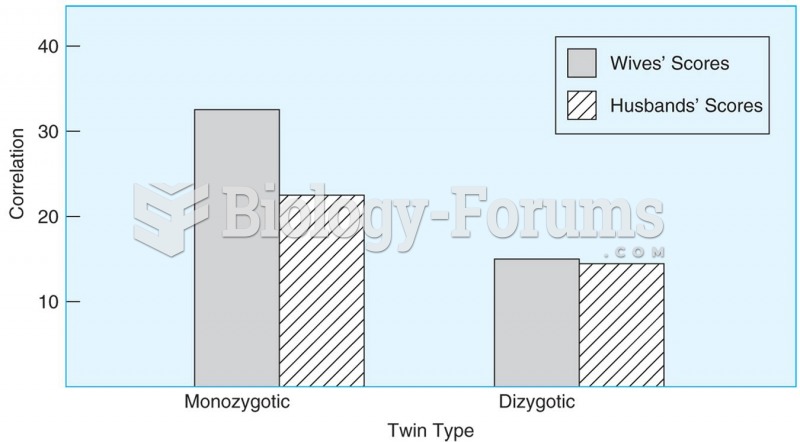
|
|
|
More than nineteen million Americans carry the factor V gene that causes blood clots, pulmonary embolism, and heart disease.
Multiple experimental evidences have confirmed that at the molecular level, cancer is caused by lesions in cellular DNA.
Walt Disney helped combat malaria by making an animated film in 1943 called The Winged Scourge. This short film starred the seven dwarfs and taught children that mosquitos transmit malaria, which is a very bad disease. It advocated the killing of mosquitos to stop the disease.
Famous people who died from poisoning or drug overdose include, Adolf Hitler, Socrates, Juan Ponce de Leon, Marilyn Monroe, Judy Garland, and John Belushi.
Human neurons are so small that they require a microscope in order to be seen. However, some neurons can be up to 3 feet long, such as those that extend from the spinal cord to the toes.
 The term wasp is typically defined as any insect of the order Hymenoptera and suborder Apocrita that
The term wasp is typically defined as any insect of the order Hymenoptera and suborder Apocrita that
 Older adults may grieve intensely over the loss of a person or situation that has been a part of the
Older adults may grieve intensely over the loss of a person or situation that has been a part of the