|
|
|
IgA antibodies protect body surfaces exposed to outside foreign substances. IgG antibodies are found in all body fluids. IgM antibodies are the first type of antibody made in response to an infection. IgE antibody levels are often high in people with allergies. IgD antibodies are found in tissues lining the abdomen and chest.
Women are 50% to 75% more likely than men to experience an adverse drug reaction.
Side effects from substance abuse include nausea, dehydration, reduced productivitiy, and dependence. Though these effects usually worsen over time, the constant need for the substance often overcomes rational thinking.
There are more sensory neurons in the tongue than in any other part of the body.
The average office desk has 400 times more bacteria on it than a toilet.
 A photograph of Commodore Matthew Perry in 1855 juxtaposed with a Japanese portrait of him. When Per
A photograph of Commodore Matthew Perry in 1855 juxtaposed with a Japanese portrait of him. When Per
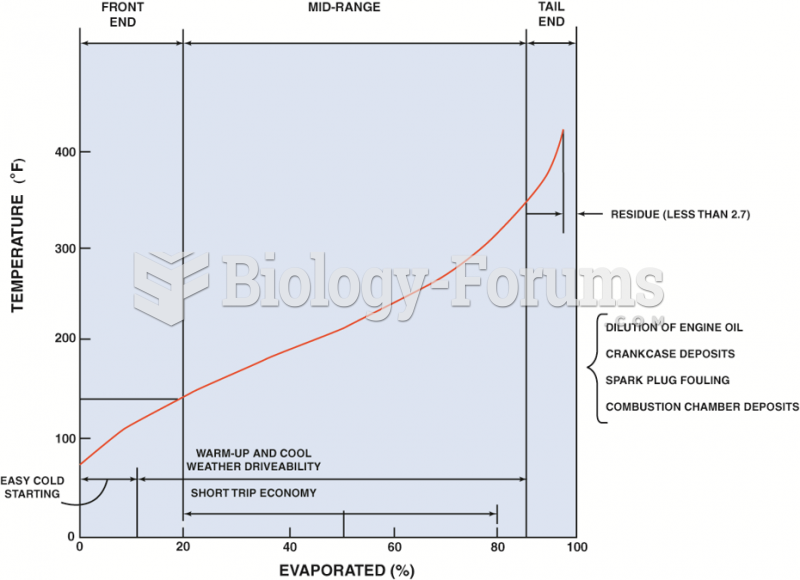 A typical distillation curve. Heavier molecules evaporate at higher temperatures and contain more ...
A typical distillation curve. Heavier molecules evaporate at higher temperatures and contain more ...





