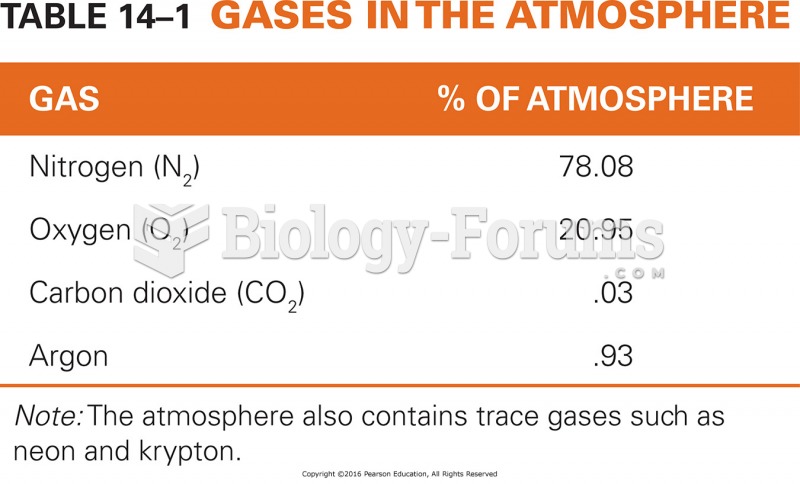
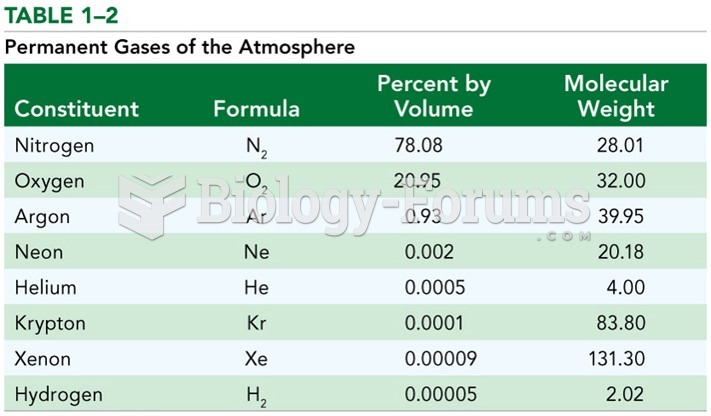
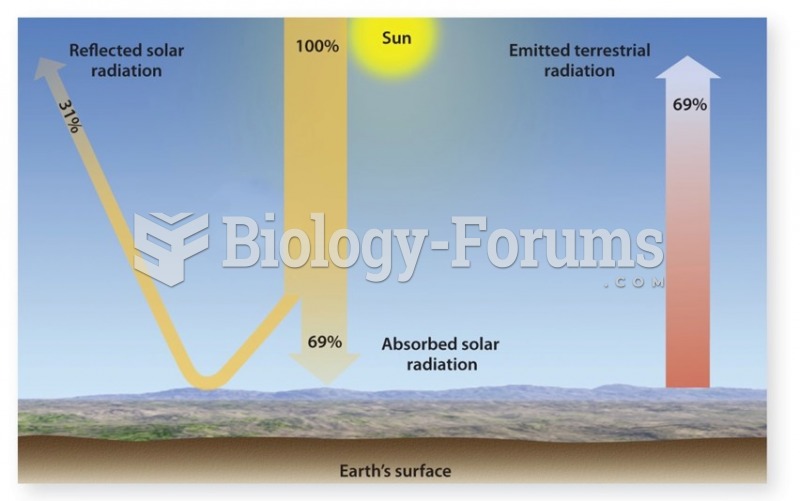
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
Did you know?
Thyroid conditions may make getting pregnant impossible.
Did you know?
All adverse reactions are commonly charted in red ink in the patient's record and usually are noted on the front of the chart. Failure to follow correct documentation procedures may result in malpractice lawsuits.
Did you know?
On average, the stomach produces 2 L of hydrochloric acid per day.
Did you know?
The most destructive flu epidemic of all times in recorded history occurred in 1918, with approximately 20 million deaths worldwide.