|
|
|
Allergies play a major part in the health of children. The most prevalent childhood allergies are milk, egg, soy, wheat, peanuts, tree nuts, and seafood.
The average adult has about 21 square feet of skin.
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
Once thought to have neurofibromatosis, Joseph Merrick (also known as "the elephant man") is now, in retrospect, thought by clinical experts to have had Proteus syndrome. This endocrine disease causes continued and abnormal growth of the bones, muscles, skin, and so on and can become completely debilitating with severe deformities occurring anywhere on the body.
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.
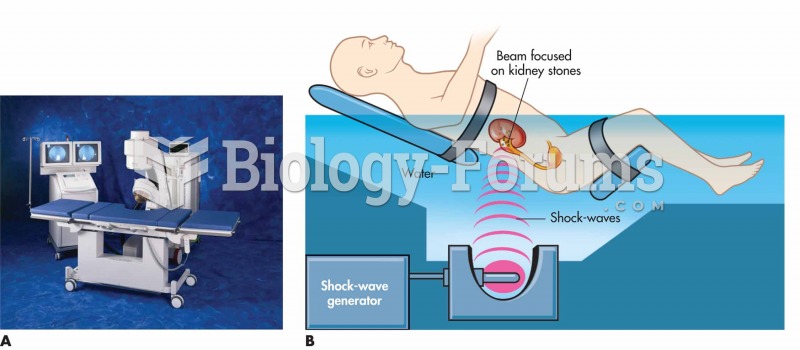 (A) Dornier Compact Delta® lithotripsy system. Acoustic shock waves generated by the shock-wave-gene
(A) Dornier Compact Delta® lithotripsy system. Acoustic shock waves generated by the shock-wave-gene
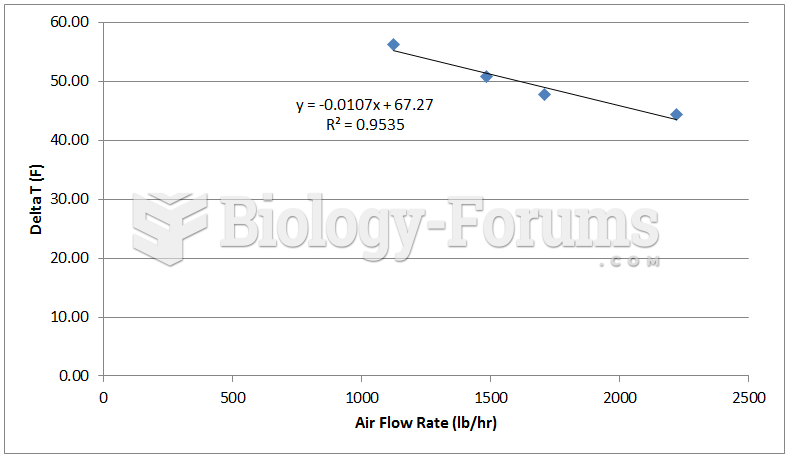
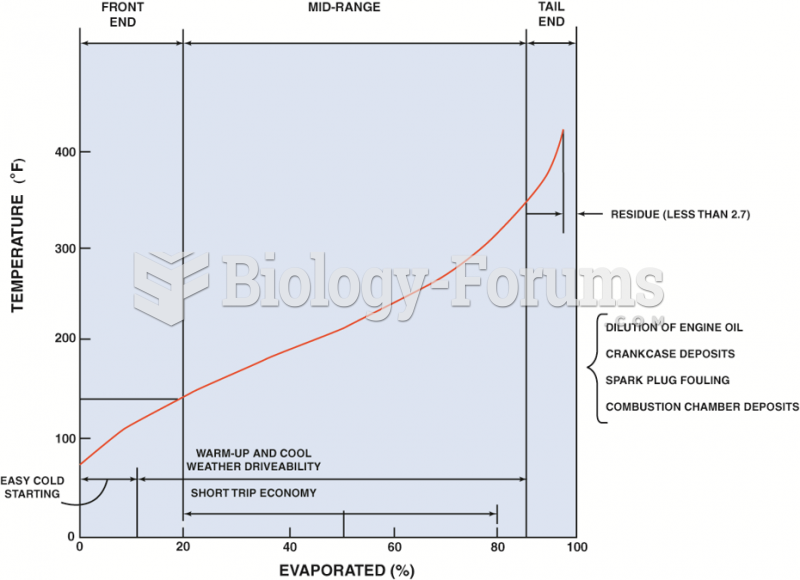 A typical distillation curve. Heavier molecules evaporate at higher temperatures and contain more ...
A typical distillation curve. Heavier molecules evaporate at higher temperatures and contain more ...