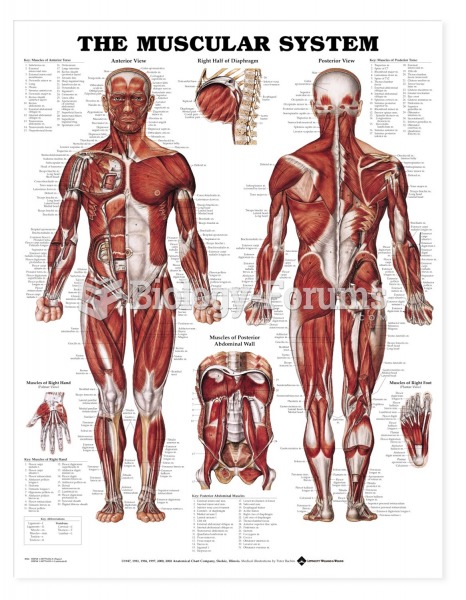
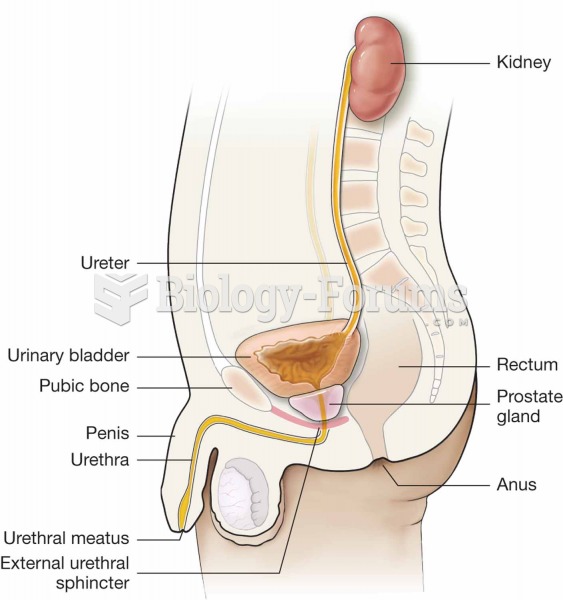
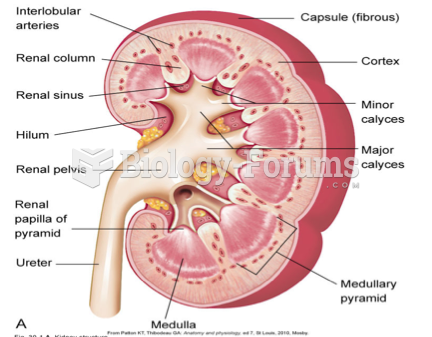
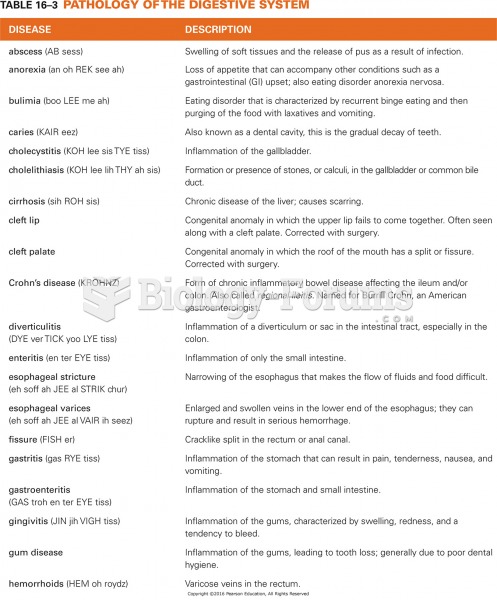
|
|
|
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.
The familiar sounds of your heart are made by the heart's valves as they open and close.
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
The top 10 most important tips that will help you grow old gracefully include (1) quit smoking, (2) keep your weight down, (3) take supplements, (4) skip a meal each day or fast 1 day per week, (5) get a pet, (6) get medical help for chronic pain, (7) walk regularly, (8) reduce arguments, (9) put live plants in your living space, and (10) do some weight training.
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.