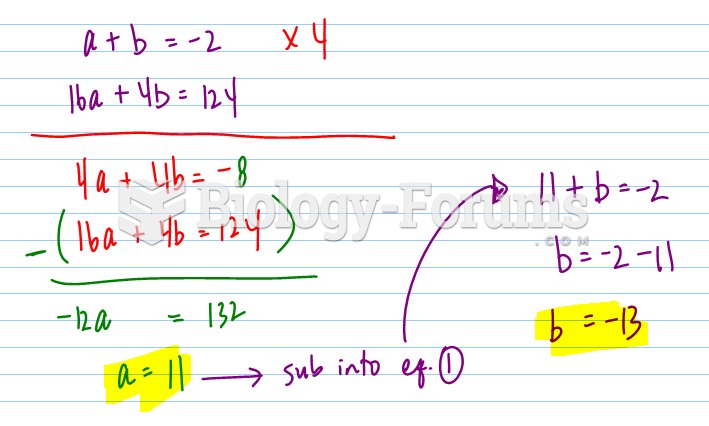
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
Did you know?
Asthma cases in Americans are about 75% higher today than they were in 1980.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
Elderly adults are living longer, and causes of death are shifting. At the same time, autopsy rates are at or near their lowest in history.
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.