This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
You should not take more than 1,000 mg of vitamin E per day. Doses above this amount increase the risk of bleeding problems that can lead to a stroke.
Did you know?
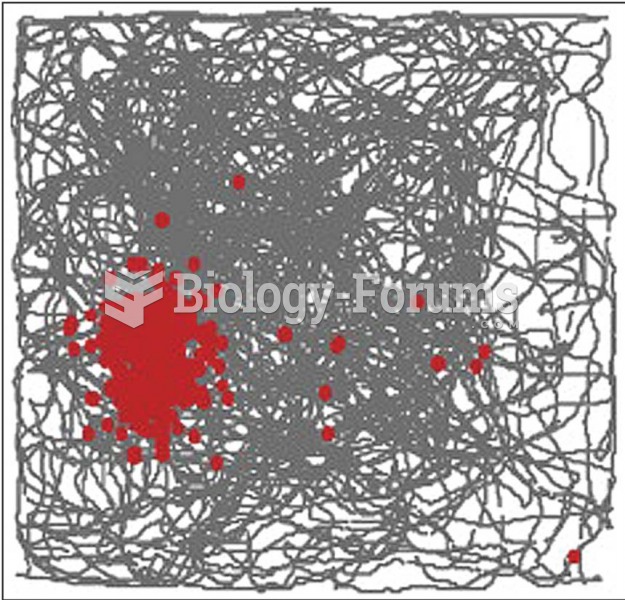
Patients who have undergone chemotherapy for the treatment of cancer often complain of a lack of mental focus; memory loss; and a general diminution in abilities such as multitasking, attention span, and general mental agility.
Did you know?
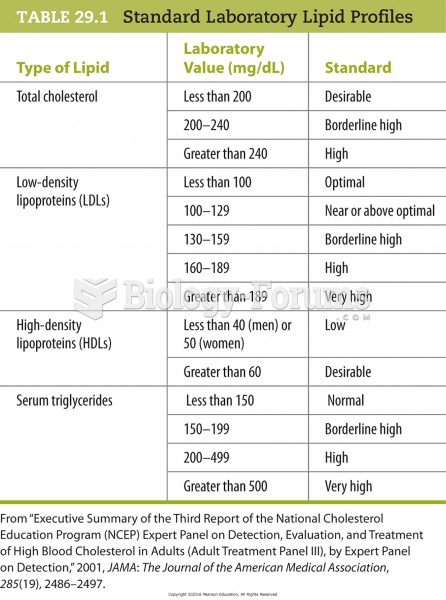
More than 150,000 Americans killed by cardiovascular disease are younger than the age of 65 years.
Did you know?
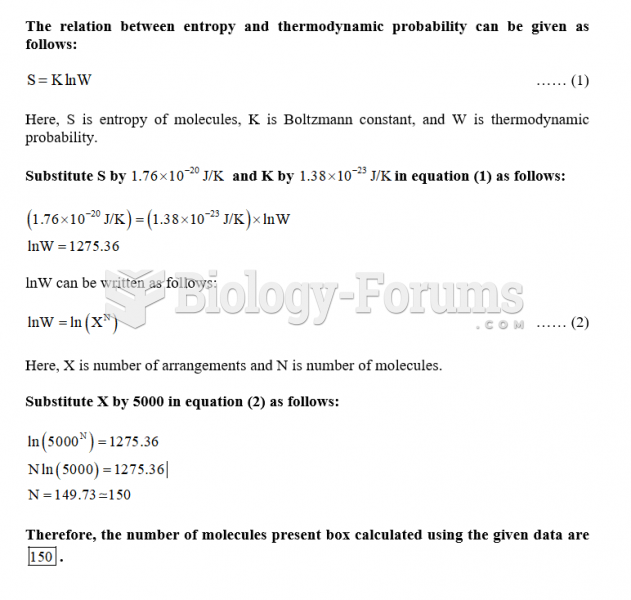
The average human gut is home to perhaps 500 to 1,000 different species of bacteria.
Did you know?
Limit intake of red meat and dairy products made with whole milk. Choose skim milk, low-fat or fat-free dairy products. Limit fried food. Use healthy oils when cooking.