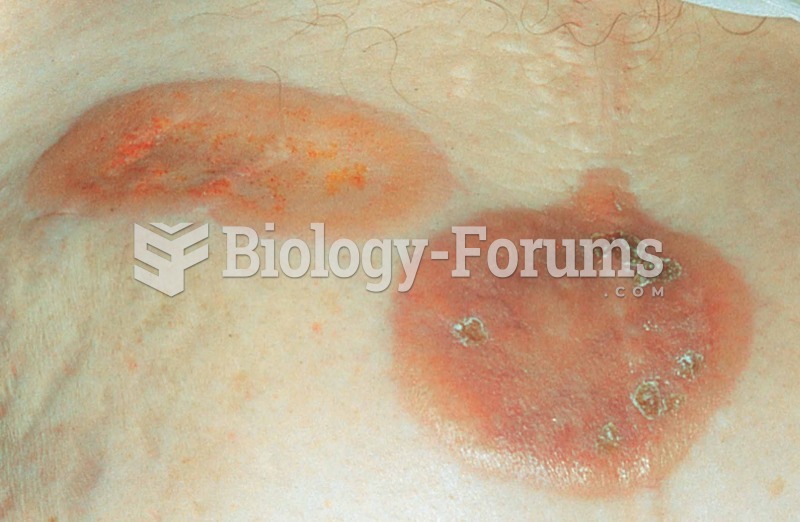
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Stroke kills people from all ethnic backgrounds, but the people at highest risk for fatal strokes are: black men, black women, Asian men, white men, and white women.
Did you know?
One way to reduce acid reflux is to lose two or three pounds. Most people lose weight in the belly area first when they increase exercise, meaning that heartburn can be reduced quickly by this method.
Did you know?
Parkinson's disease is both chronic and progressive. This means that it persists over a long period of time and that its symptoms grow worse over time.
Did you know?
About 100 new prescription or over-the-counter drugs come into the U.S. market every year.
Did you know?
On average, the stomach produces 2 L of hydrochloric acid per day.