This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
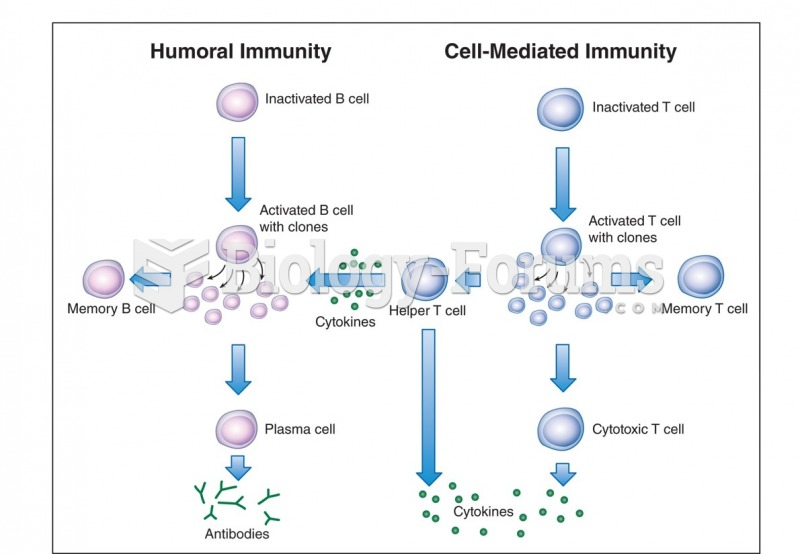
Autoimmune diseases occur when the immune system destroys its own healthy tissues. When this occurs, white blood cells cannot distinguish between pathogens and normal cells.
Did you know?
Cyanide works by making the human body unable to use oxygen.
Did you know?
No drugs are available to relieve parathyroid disease. Parathyroid disease is caused by a parathyroid tumor, and it needs to be removed by surgery.
Did you know?
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.