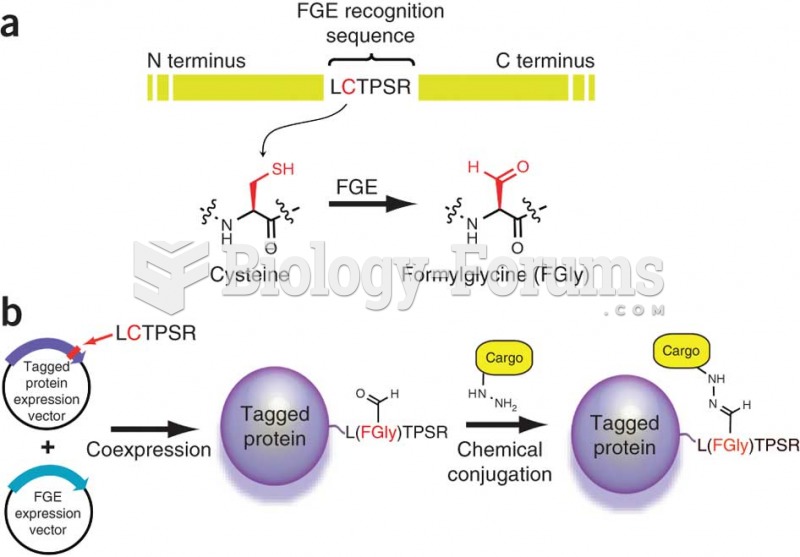
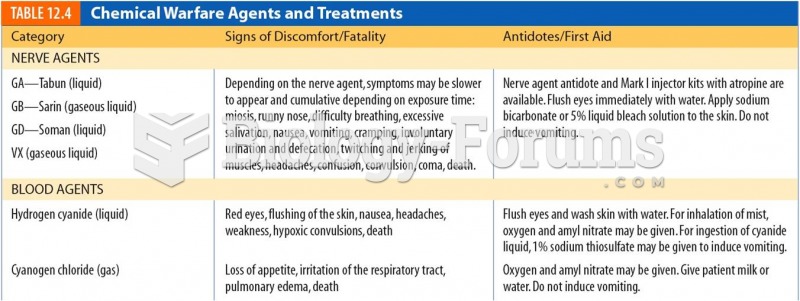
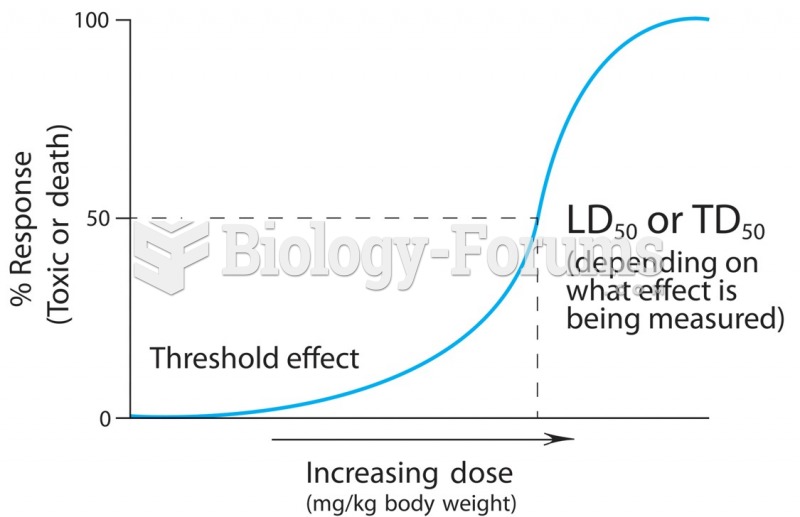
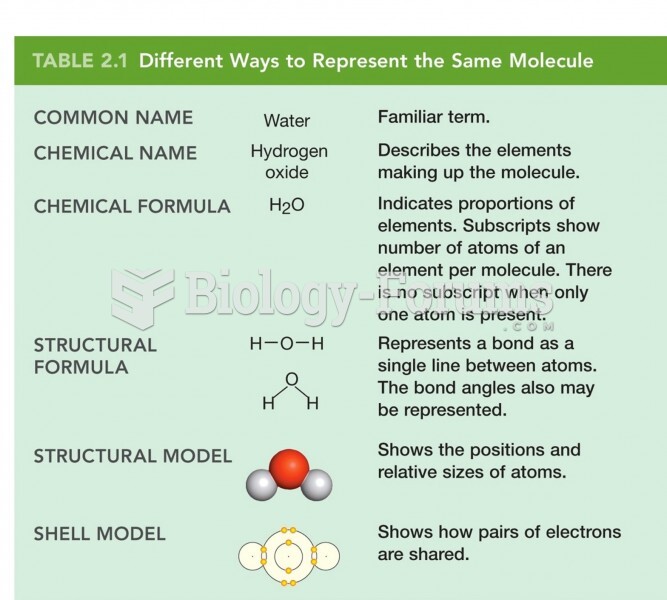
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.
Did you know?
The first documented use of surgical anesthesia in the United States was in Connecticut in 1844.
Did you know?
It is believed that humans initially contracted crabs from gorillas about 3 million years ago from either sleeping in gorilla nests or eating the apes.
Did you know?
Green tea is able to stop the scent of garlic or onion from causing bad breath.
Did you know?
By definition, when a medication is administered intravenously, its bioavailability is 100%.