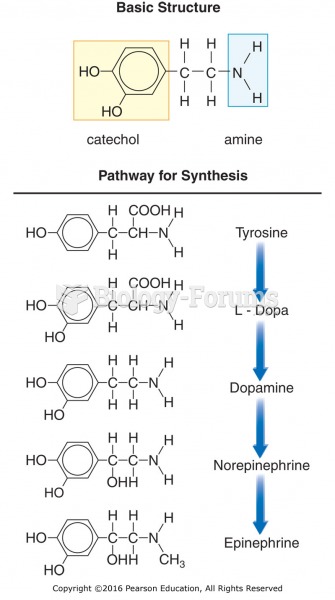
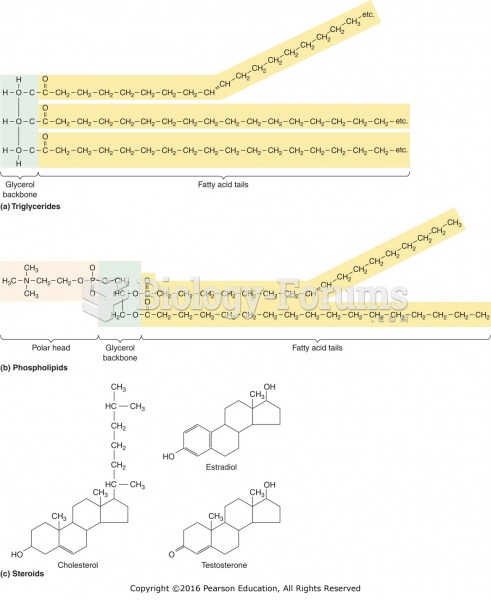
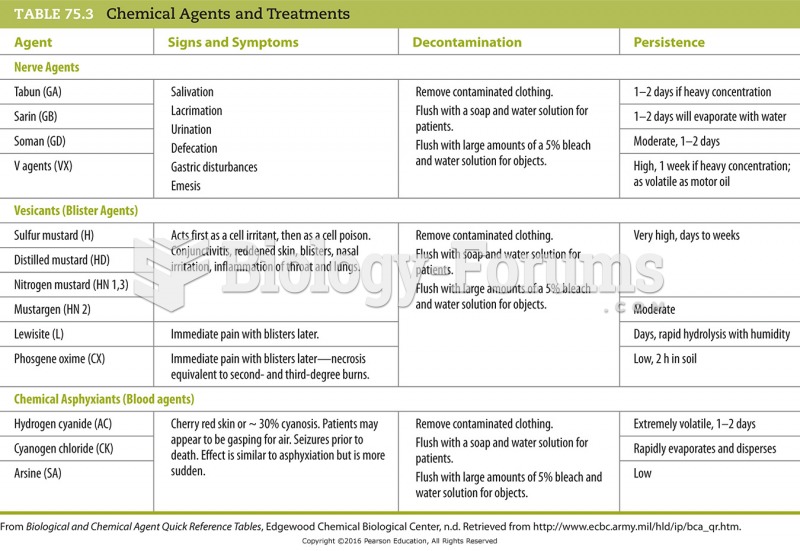
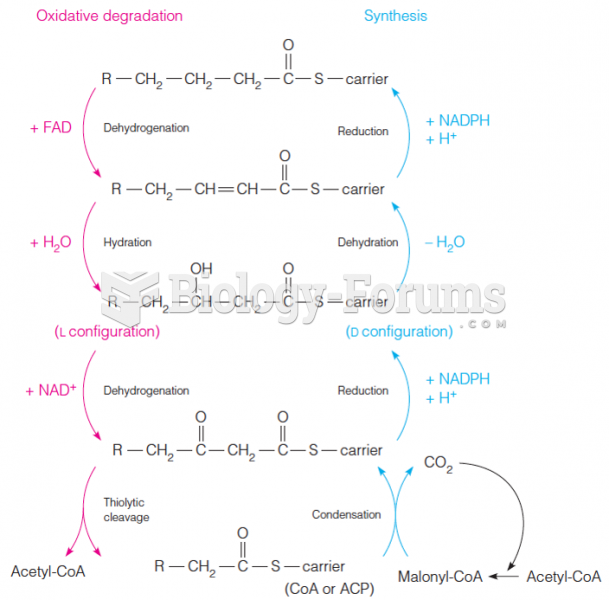
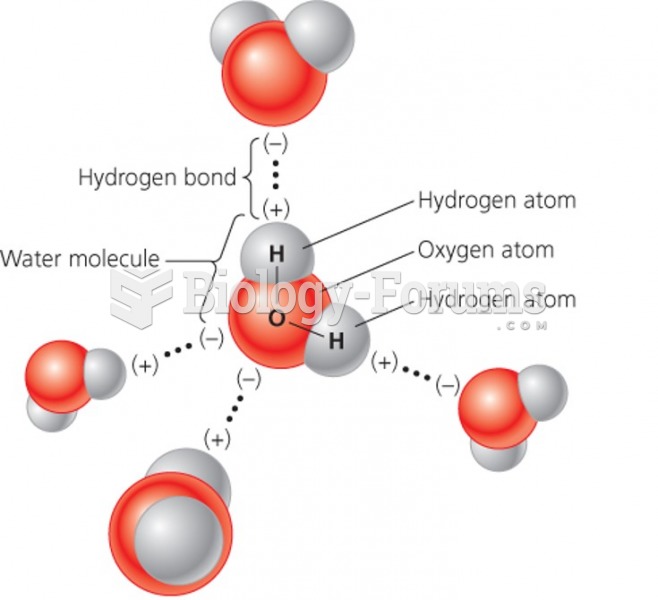
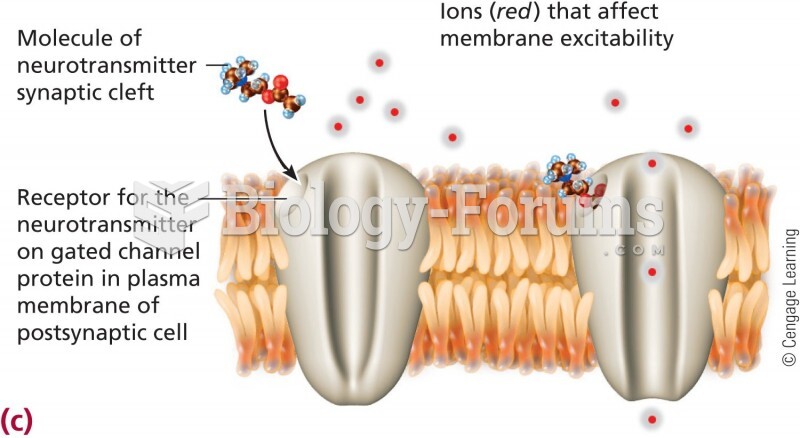
|
|
|
The liver is the only organ that has the ability to regenerate itself after certain types of damage. As much as 25% of the liver can be removed, and it will still regenerate back to its original shape and size. However, the liver cannot regenerate after severe damage caused by alcohol.
More than 2,500 barbiturates have been synthesized. At the height of their popularity, about 50 were marketed for human use.
The first oncogene was discovered in 1970 and was termed SRC (pronounced "SARK").
Aspirin is the most widely used drug in the world. It has even been recognized as such by the Guinness Book of World Records.
Intradermal injections are somewhat difficult to correctly administer because the skin layers are so thin that it is easy to accidentally punch through to the deeper subcutaneous layer.