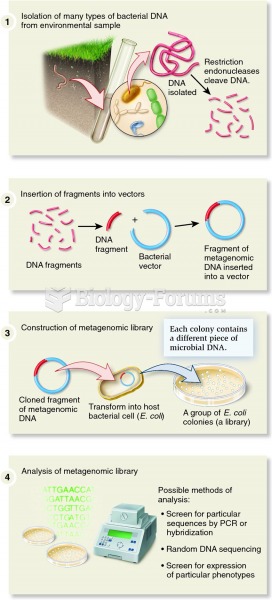
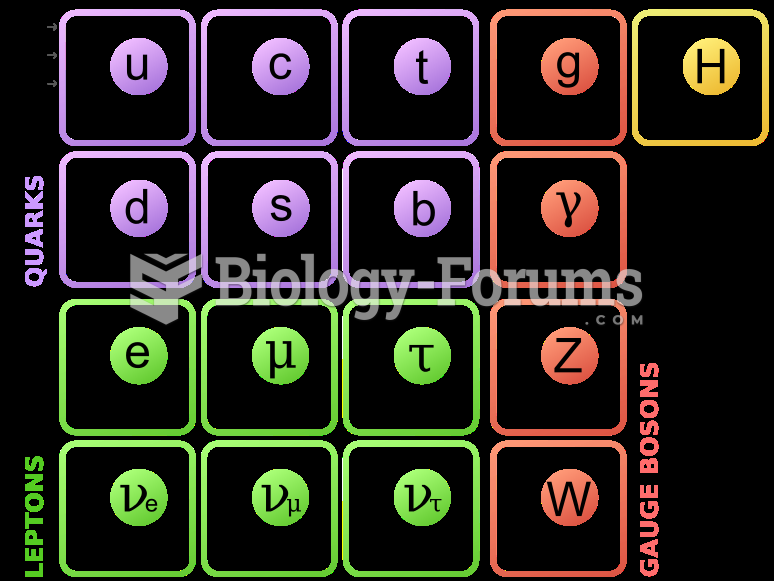
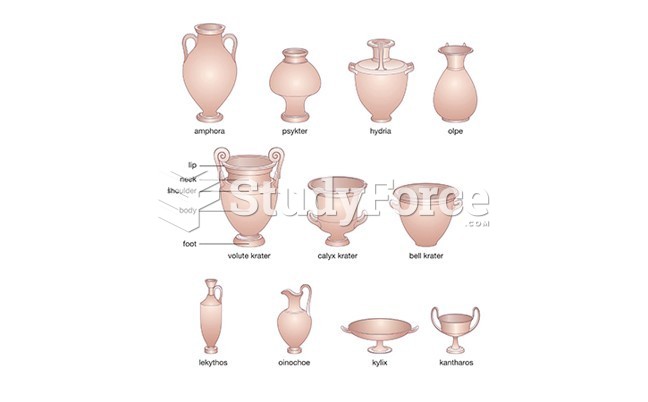
|
|
|
Most fungi that pathogenically affect humans live in soil. If a person is not healthy, has an open wound, or is immunocompromised, a fungal infection can be very aggressive.
During the twentieth century, a variant of the metric system was used in Russia and France in which the base unit of mass was the tonne. Instead of kilograms, this system used millitonnes (mt).
Bacteria have flourished on the earth for over three billion years. They were the first life forms on the planet.
Patients who cannot swallow may receive nutrition via a parenteral route—usually, a catheter is inserted through the chest into a large vein going into the heart.
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.