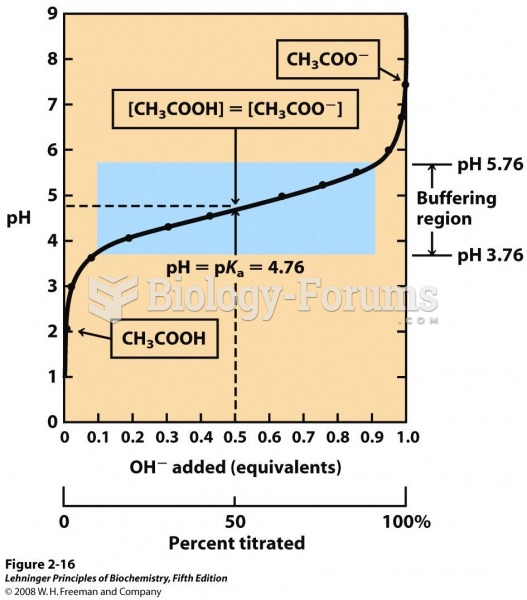
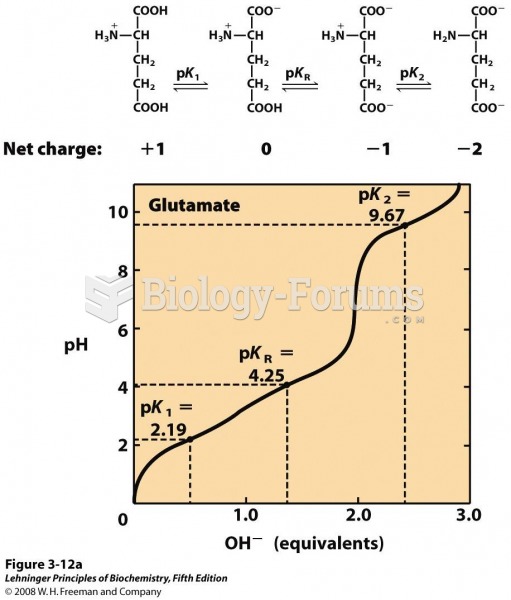
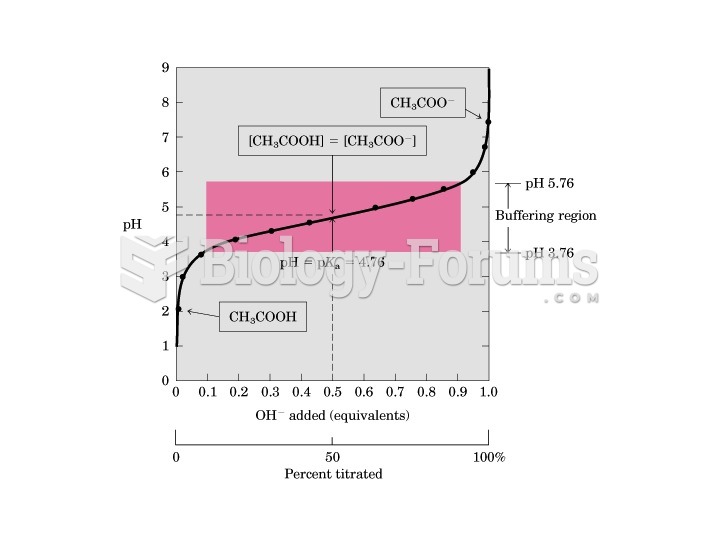
|
|
|
People with alcoholism are at a much greater risk of malnutrition than are other people and usually exhibit low levels of most vitamins (especially folic acid). This is because alcohol often takes the place of 50% of their daily intake of calories, with little nutritional value contained in it.
Essential fatty acids have been shown to be effective against ulcers, asthma, dental cavities, and skin disorders such as acne.
Pubic lice (crabs) are usually spread through sexual contact. You cannot catch them by using a public toilet.
Earwax has antimicrobial properties that reduce the viability of bacteria and fungus in the human ear.
Malaria was not eliminated in the United States until 1951. The term eliminated means that no new cases arise in a country for 3 years.