|
|
|
Studies show that systolic blood pressure can be significantly lowered by taking statins. In fact, the higher the patient's baseline blood pressure, the greater the effect of statins on his or her blood pressure.
Signs and symptoms of a drug overdose include losing consciousness, fever or sweating, breathing problems, abnormal pulse, and changes in skin color.
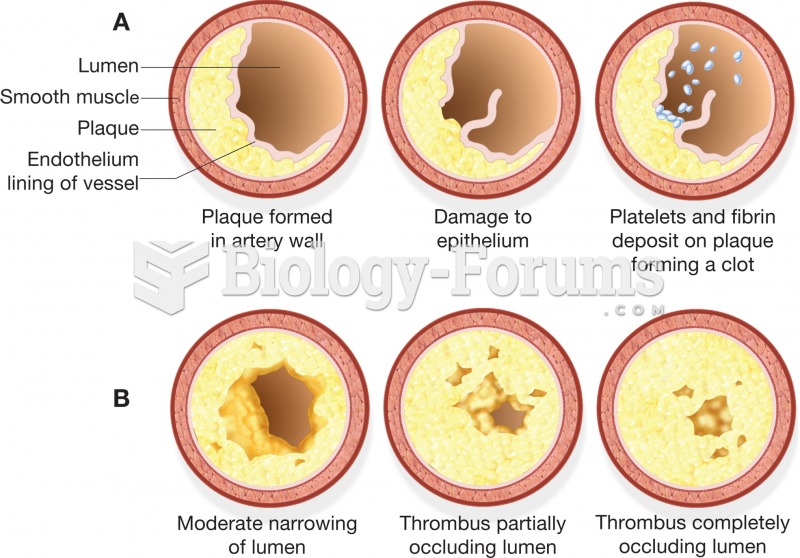
When blood is exposed to air, it clots. Heparin allows the blood to come in direct contact with air without clotting.
In most climates, 8 to 10 glasses of water per day is recommended for adults. The best indicator for adequate fluid intake is frequent, clear urination.
Walt Disney helped combat malaria by making an animated film in 1943 called The Winged Scourge. This short film starred the seven dwarfs and taught children that mosquitos transmit malaria, which is a very bad disease. It advocated the killing of mosquitos to stop the disease.





![What is the pH of an aqueous solution if the [H+] = 0.000 000 075 M?](https://biology-forums.com/gallery/43/medium_6_11_12_21_7_23_55.jpeg)