This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
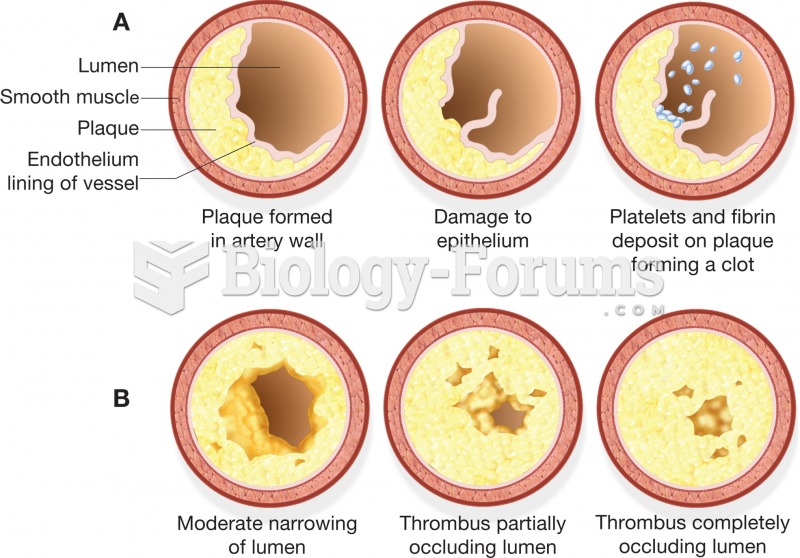
On average, someone in the United States has a stroke about every 40 seconds. This is about 795,000 people per year.
Did you know?
The newest statin drug, rosuvastatin, has been called a superstatin because it appears to reduce LDL cholesterol to a greater degree than the other approved statin drugs.
Did you know?
Recent studies have shown that the number of medication errors increases in relation to the number of orders that are verified per pharmacist, per work shift.
Did you know?
Many of the drugs used by neuroscientists are derived from toxic plants and venomous animals (such as snakes, spiders, snails, and puffer fish).
Did you know?
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.