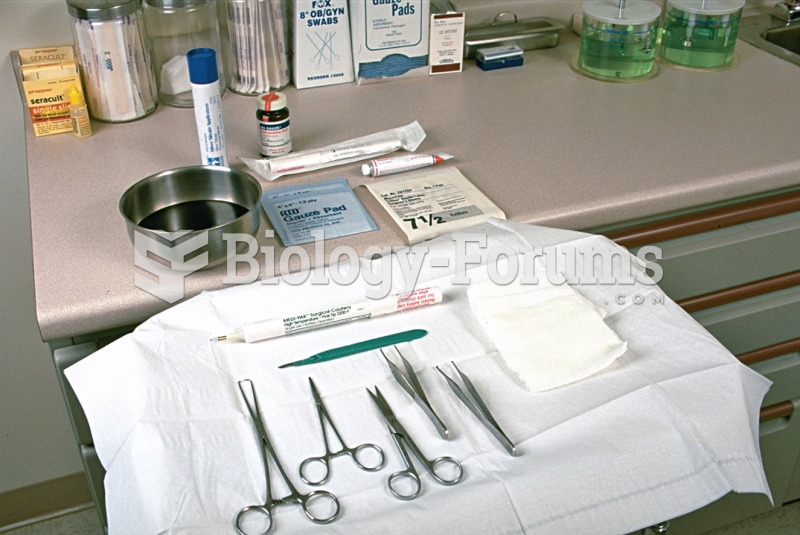
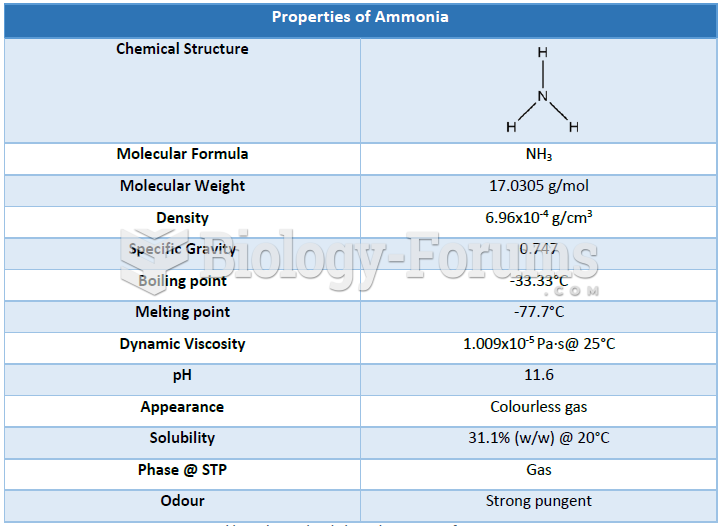
|
|
|
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
Medication errors are more common among seriously ill patients than with those with minor conditions.
The first oral chemotherapy drug for colon cancer was approved by FDA in 2001.
Signs and symptoms that may signify an eye tumor include general blurred vision, bulging eye(s), double vision, a sensation of a foreign body in the eye(s), iris defects, limited ability to move the eyelid(s), limited ability to move the eye(s), pain or discomfort in or around the eyes or eyelids, red or pink eyes, white or cloud spots on the eye(s), colored spots on the eyelid(s), swelling around the eyes, swollen eyelid(s), and general vision loss.
More than 30% of American adults, and about 12% of children utilize health care approaches that were developed outside of conventional medicine.