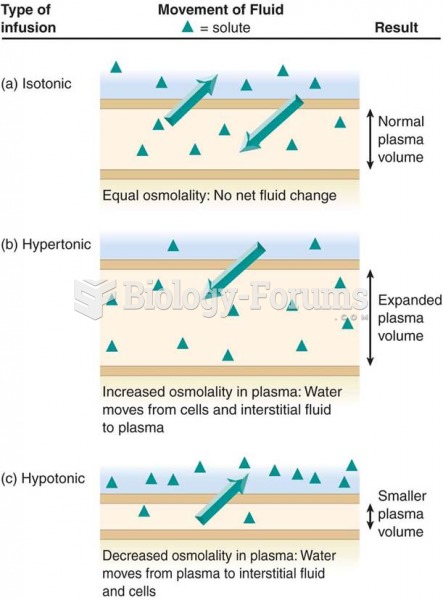
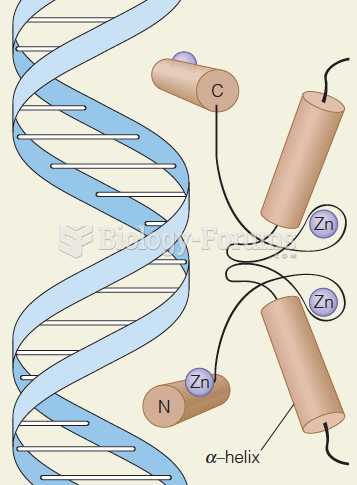
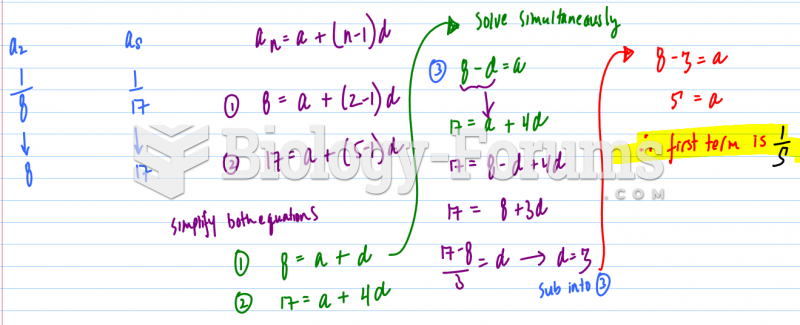
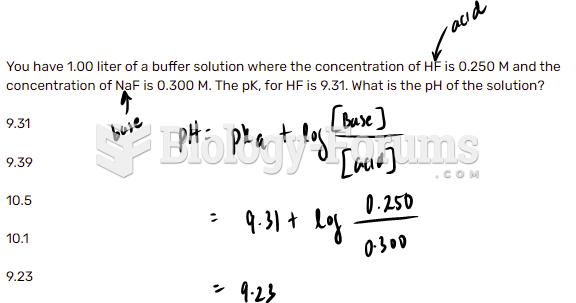
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Medication errors are more common among seriously ill patients than with those with minor conditions.
Did you know?
Certain rare plants containing cyanide include apricot pits and a type of potato called cassava. Fortunately, only chronic or massive ingestion of any of these plants can lead to serious poisoning.
Did you know?
Most childhood vaccines are 90–99% effective in preventing disease. Side effects are rarely serious.
Did you know?
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.
Did you know?
Despite claims by manufacturers, the supplement known as Ginkgo biloba was shown in a study of more than 3,000 participants to be ineffective in reducing development of dementia and Alzheimer’s disease in older people.