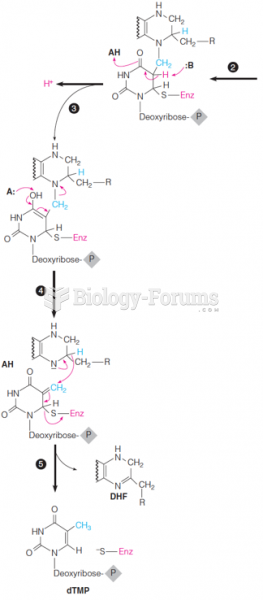
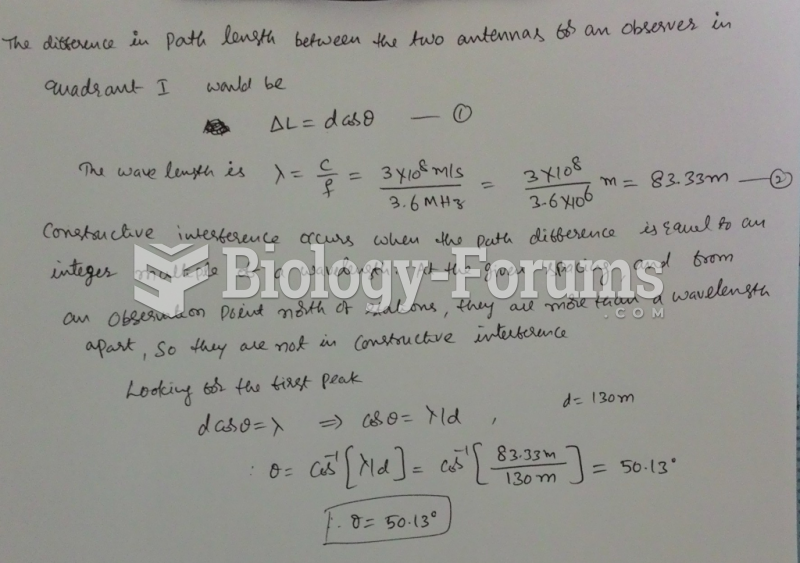
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Alzheimer's disease affects only about 10% of people older than 65 years of age. Most forms of decreased mental function and dementia are caused by disuse (letting the mind get lazy).
Did you know?
Approximately 500,000 babies are born each year in the United States to teenage mothers.
Did you know?
There are 60,000 miles of blood vessels in every adult human.
Did you know?
The FDA recognizes 118 routes of administration.
Did you know?
About 600,000 particles of skin are shed every hour by each human. If you live to age 70 years, you have shed 105 pounds of dead skin.