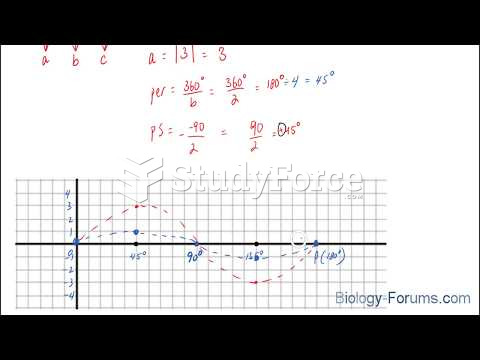
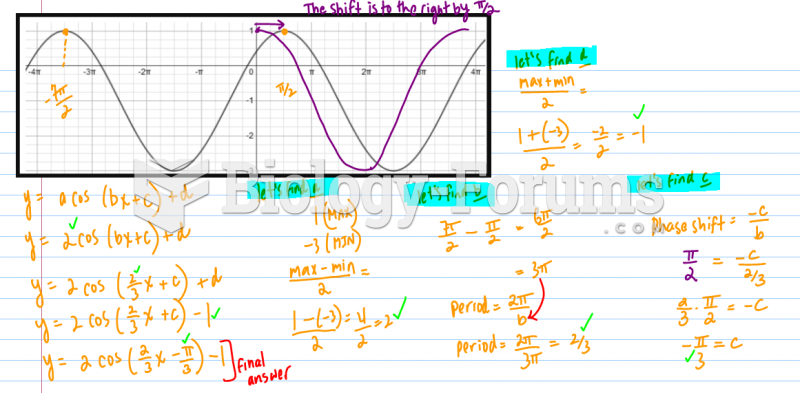
|
|
|
The U.S. Preventive Services Task Force recommends that all women age 65 years of age or older should be screened with bone densitometry.
Warfarin was developed as a consequence of the study of a strange bleeding disorder that suddenly occurred in cattle on the northern prairies of the United States in the early 1900s.
Russia has the highest death rate from cardiovascular disease followed by the Ukraine, Romania, Hungary, and Poland.
If you use artificial sweeteners, such as cyclamates, your eyes may be more sensitive to light. Other factors that will make your eyes more sensitive to light include use of antibiotics, oral contraceptives, hypertension medications, diuretics, and antidiabetic medications.
After 5 years of being diagnosed with rheumatoid arthritis, one every three patients will no longer be able to work.