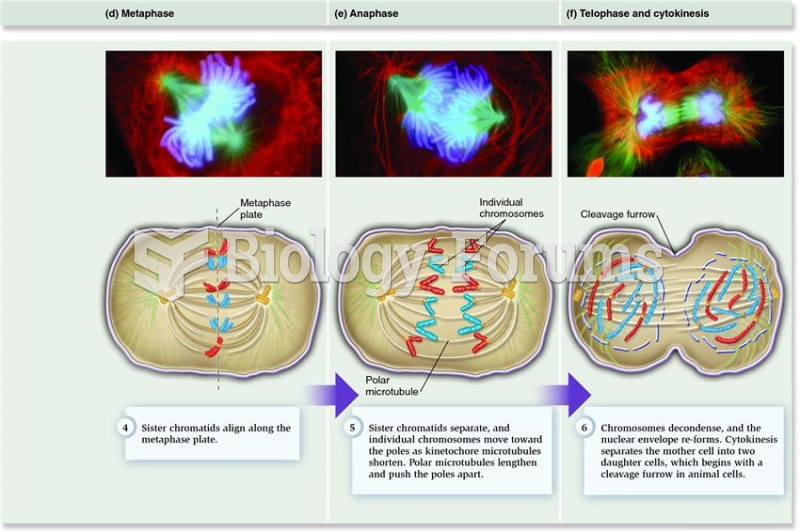
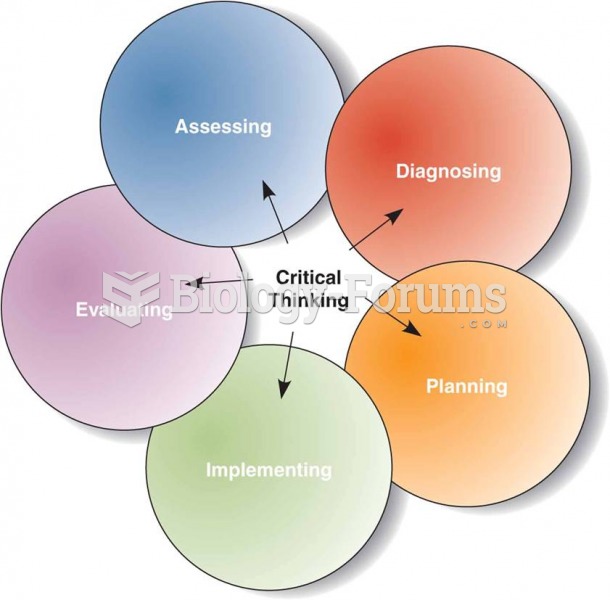
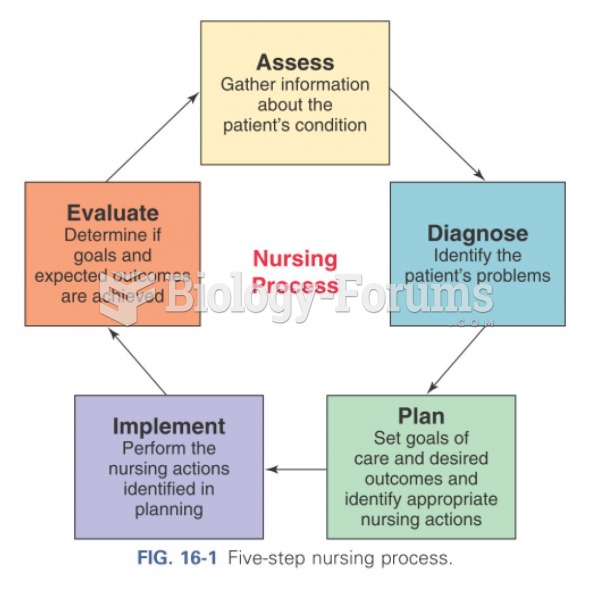
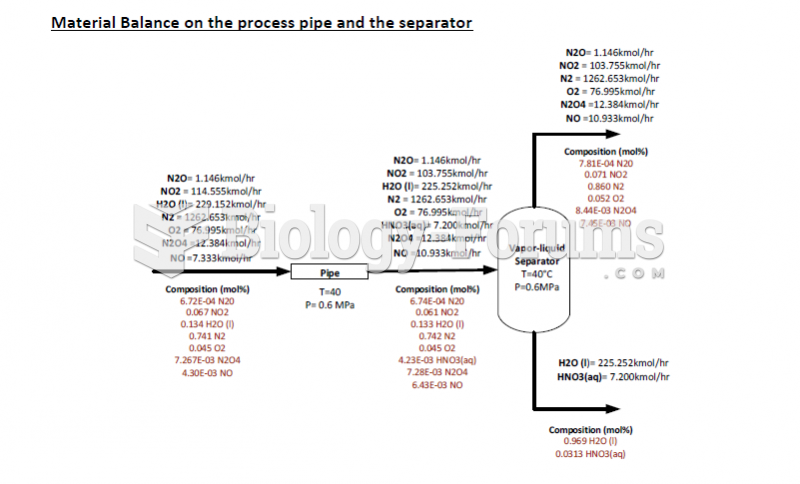
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Aspirin may benefit 11 different cancers, including those of the colon, pancreas, lungs, prostate, breasts, and leukemia.
Did you know?
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
Did you know?
In 1864, the first barbiturate (barbituric acid) was synthesized.
Did you know?
The shortest mature adult human of whom there is independent evidence was Gul Mohammed in India. In 1990, he was measured in New Delhi and stood 22.5 inches tall.
Did you know?
The Centers for Disease Control and Prevention (CDC) was originally known as the Communicable Disease Center, which was formed to fight malaria. It was originally headquartered in Atlanta, Georgia, since the Southern states faced the worst threat from malaria.