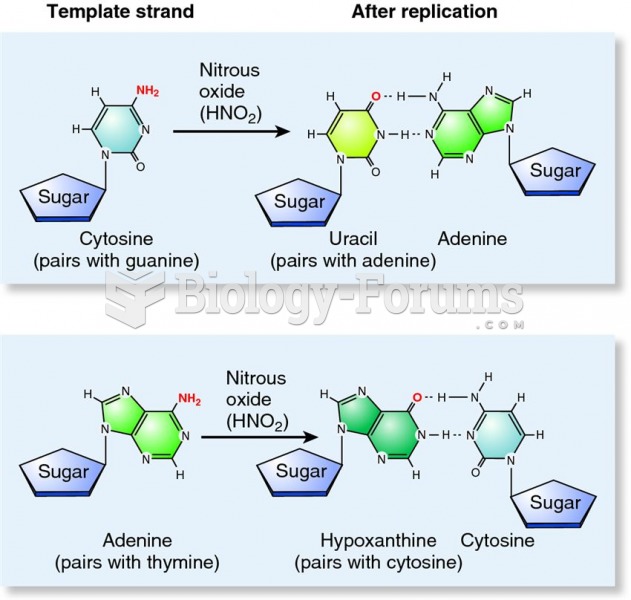
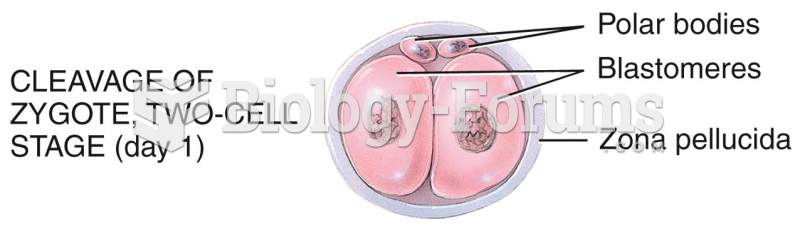
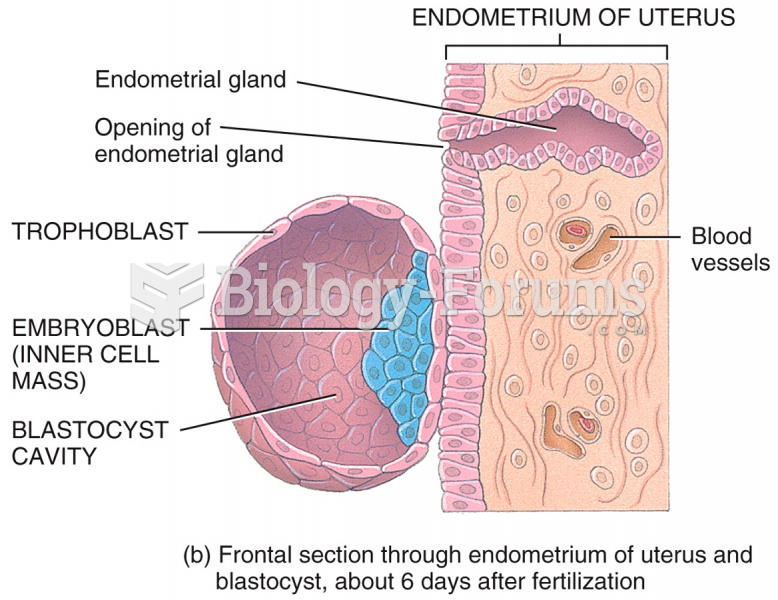
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Signs and symptoms of a drug overdose include losing consciousness, fever or sweating, breathing problems, abnormal pulse, and changes in skin color.
Did you know?
Since 1988, the CDC has reported a 99% reduction in bacterial meningitis caused by Haemophilus influenzae, due to the introduction of the vaccine against it.
Did you know?
In 2010, opiate painkllers, such as morphine, OxyContin®, and Vicodin®, were tied to almost 60% of drug overdose deaths.
Did you know?
In the United States, an estimated 50 million unnecessary antibiotics are prescribed for viral respiratory infections.
Did you know?
The human body produces and destroys 15 million blood cells every second.