|
|
|
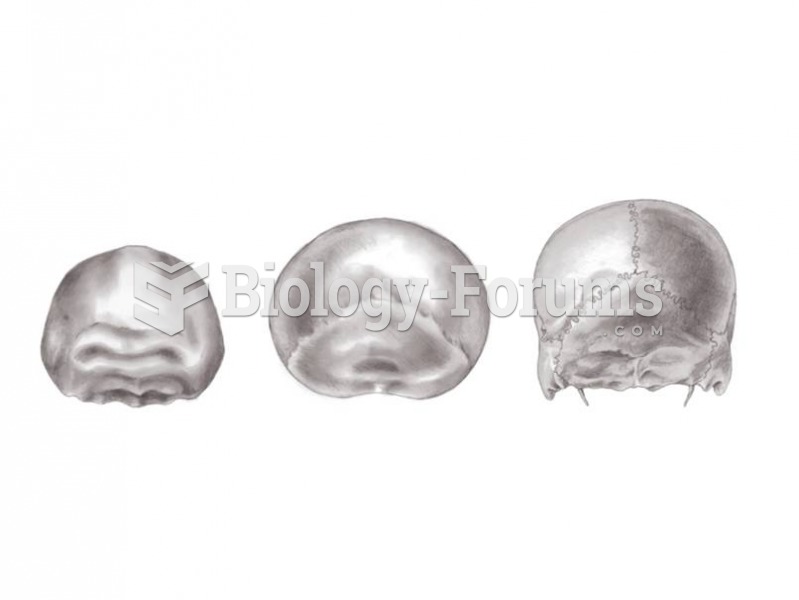
The tallest man ever known was Robert Wadlow, an American, who reached the height of 8 feet 11 inches. He died at age 26 years from an infection caused by the immense weight of his body (491 pounds) and the stress on his leg bones and muscles.
There are actually 60 minerals, 16 vitamins, 12 essential amino acids, and three essential fatty acids that your body needs every day.
The National Institutes of Health have supported research into acupuncture. This has shown that acupuncture significantly reduced pain associated with osteoarthritis of the knee, when used as a complement to conventional therapies.
Drying your hands with a paper towel will reduce the bacterial count on your hands by 45–60%.
Calcitonin is a naturally occurring hormone. In women who are at least 5 years beyond menopause, it slows bone loss and increases spinal bone density.
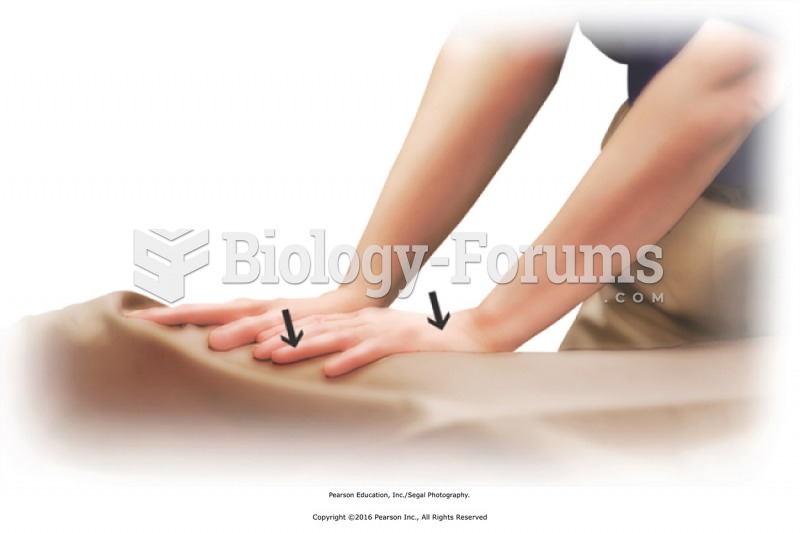 Transition from the lower to the upper limbs with compression over the drape. Apply compression with ...
Transition from the lower to the upper limbs with compression over the drape. Apply compression with ...
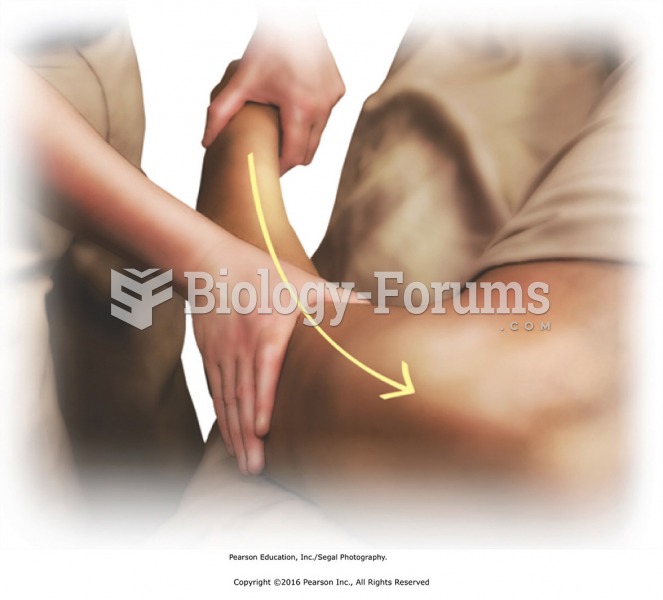 Effleurage to entire arm to reconnect and transition to the chest. Apply effleurage with moderate ...
Effleurage to entire arm to reconnect and transition to the chest. Apply effleurage with moderate ...