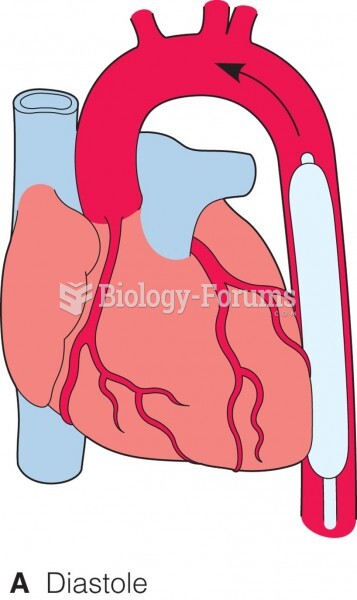
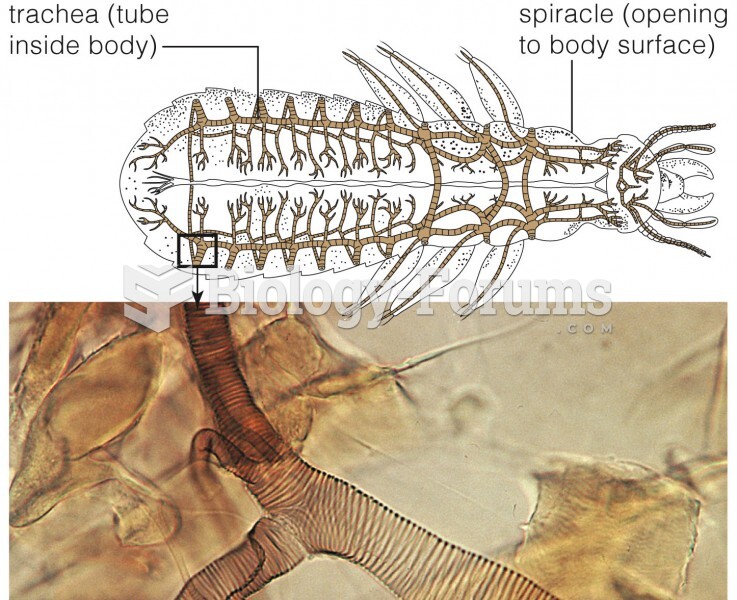
|
|
|
According to animal studies, the typical American diet is damaging to the liver and may result in allergies, low energy, digestive problems, and a lack of ability to detoxify harmful substances.
Women are two-thirds more likely than men to develop irritable bowel syndrome. This may be attributable to hormonal changes related to their menstrual cycles.
The modern decimal position system was the invention of the Hindus (around 800 AD), involving the placing of numerals to indicate their value (units, tens, hundreds, and so on).
The term pharmacology is derived from the Greek words pharmakon("claim, medicine, poison, or remedy") and logos ("study").
Over time, chronic hepatitis B virus and hepatitis C virus infections can progress to advanced liver disease, liver failure, and hepatocellular carcinoma. Unlike other forms, more than 80% of hepatitis C infections become chronic and lead to liver disease. When combined with hepatitis B, hepatitis C now accounts for 75% percent of all cases of liver disease around the world. Liver failure caused by hepatitis C is now leading cause of liver transplants in the United States.
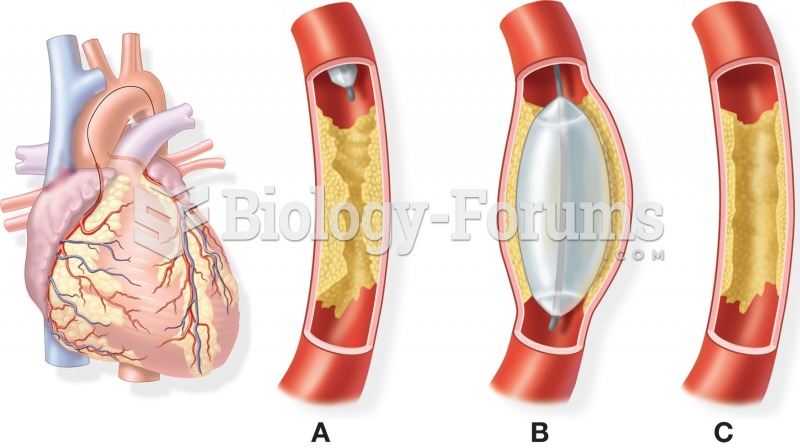 Balloon angioplasty: (A) deflated balloon catheter is approaching an atherosclerotic plaque; (B) pla
Balloon angioplasty: (A) deflated balloon catheter is approaching an atherosclerotic plaque; (B) pla
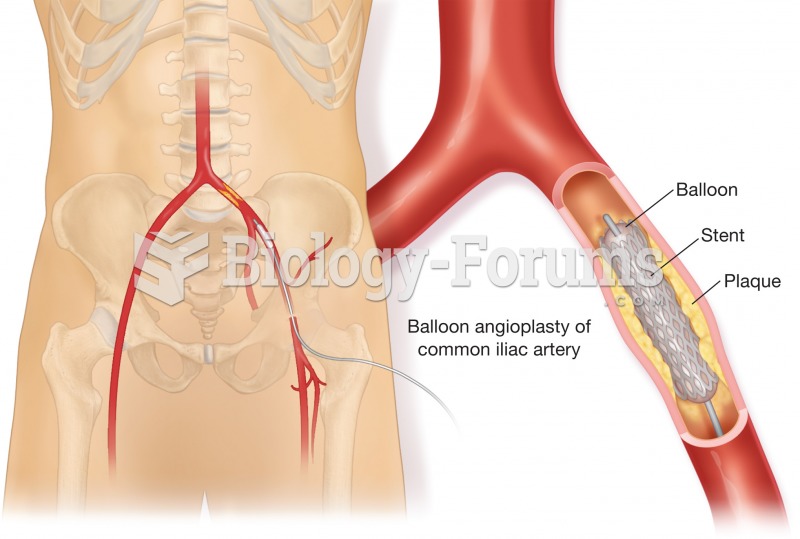 Angioplasty. One form is called balloon angioplasty, shown here. A balloon catheter is threaded into
Angioplasty. One form is called balloon angioplasty, shown here. A balloon catheter is threaded into