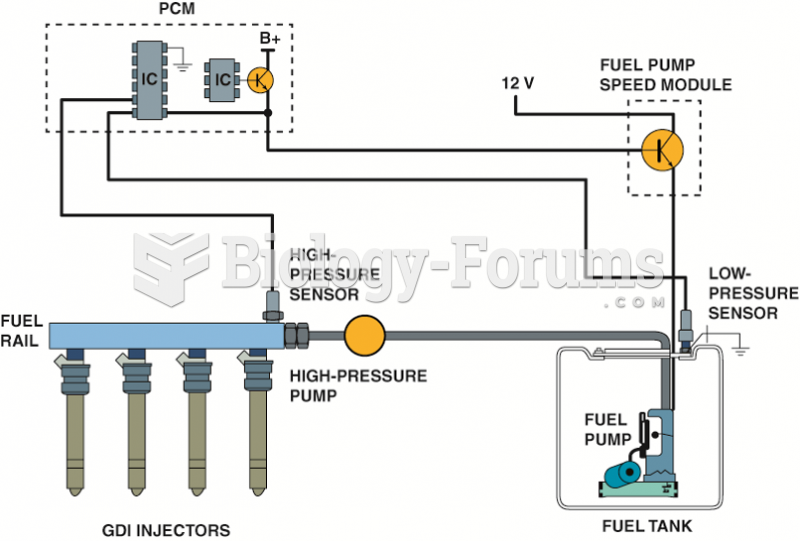
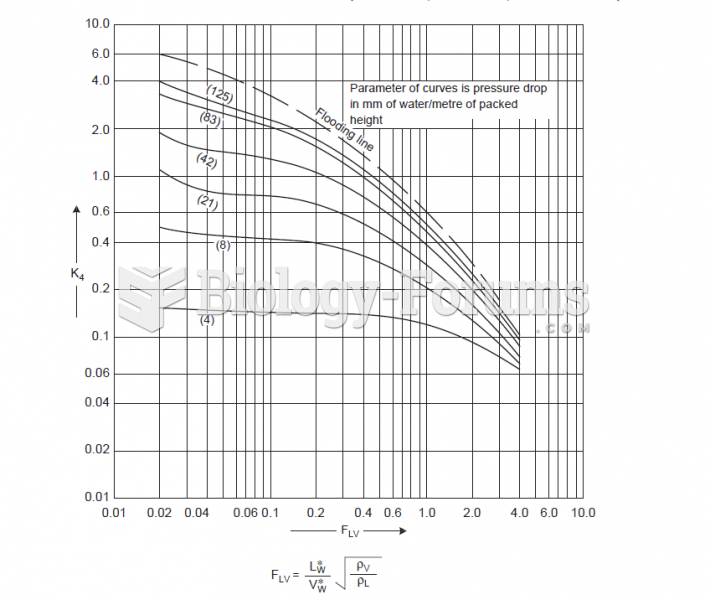
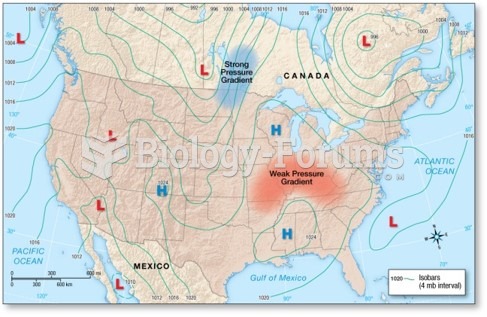
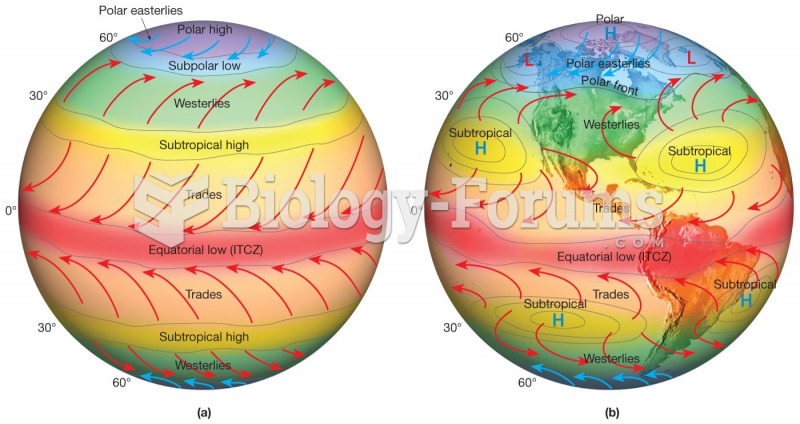
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
As the western states of America were settled, pioneers often had to drink rancid water from ponds and other sources. This often resulted in chronic diarrhea, causing many cases of dehydration and death that could have been avoided if clean water had been available.
Did you know?
More than 34,000 trademarked medication names and more than 10,000 generic medication names are in use in the United States.
Did you know?
The immune system needs 9.5 hours of sleep in total darkness to recharge completely.
Did you know?
There are 20 feet of blood vessels in each square inch of human skin.