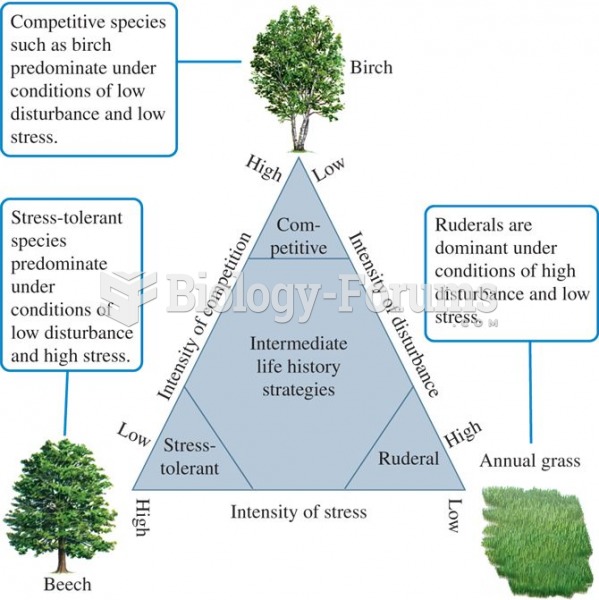
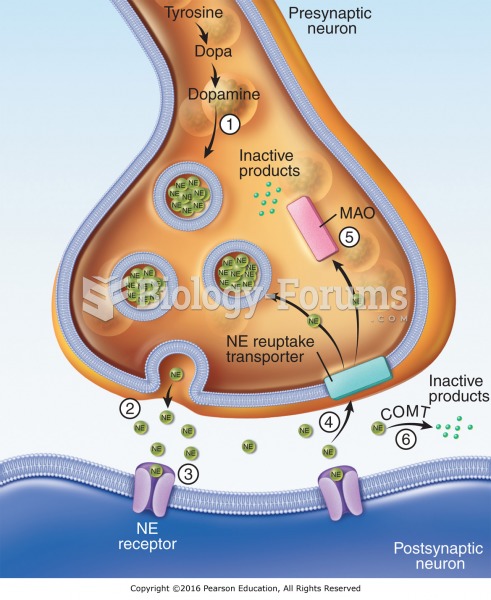
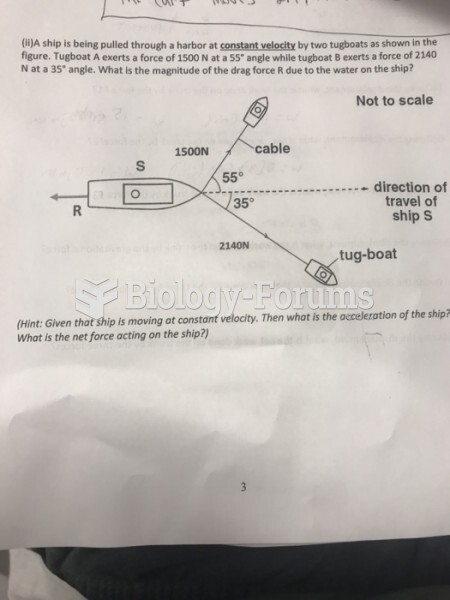
This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
The longest a person has survived after a heart transplant is 24 years.
Did you know?
Every 10 seconds, a person in the United States goes to the emergency room complaining of head pain. About 1.2 million visits are for acute migraine attacks.
Did you know?
About 3% of all pregnant women will give birth to twins, which is an increase in rate of nearly 60% since the early 1980s.
Did you know?
According to the FDA, adverse drug events harmed or killed approximately 1,200,000 people in the United States in the year 2015.
Did you know?
The most common treatment options for addiction include psychotherapy, support groups, and individual counseling.