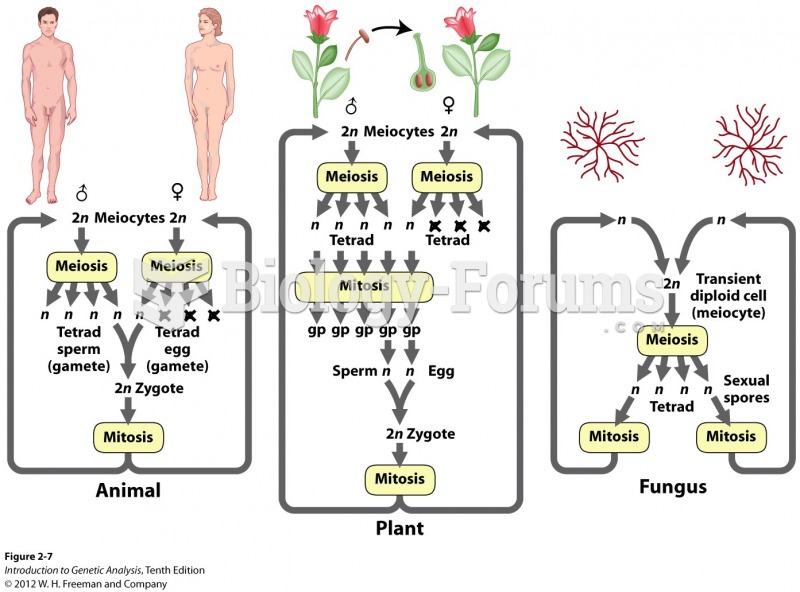
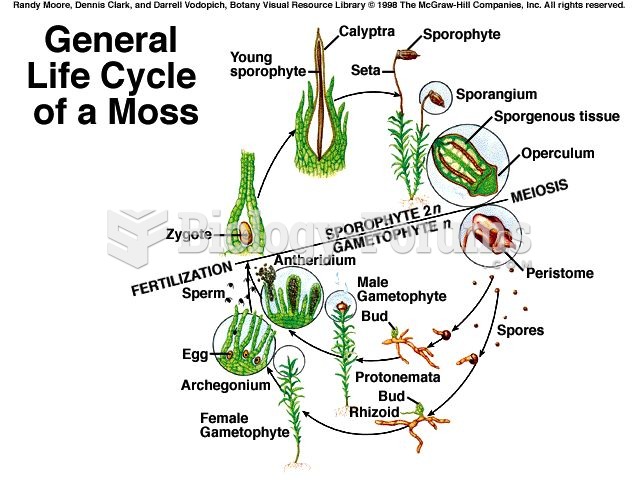
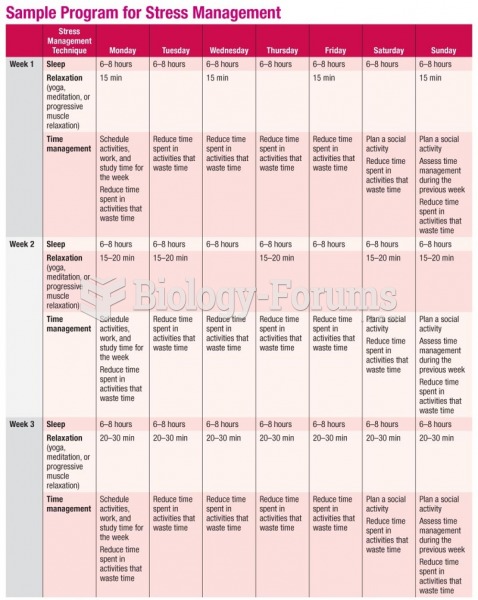
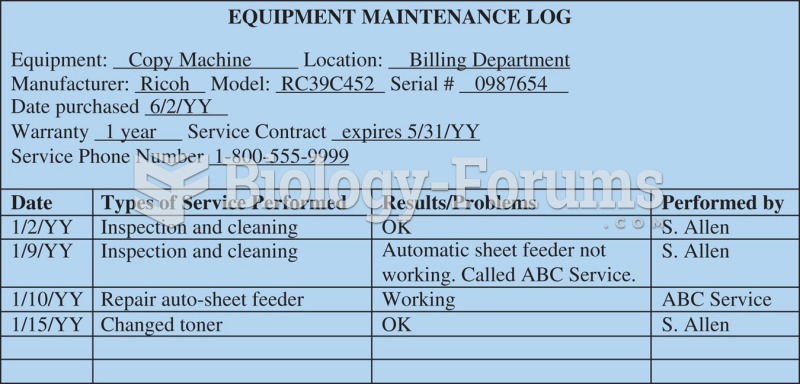
|
|
|
In Eastern Europe and Russia, interferon is administered intranasally in varied doses for the common cold and influenza. It is claimed that this treatment can lower the risk of infection by as much as 60–70%.
The human body produces and destroys 15 million blood cells every second.
Warfarin was developed as a consequence of the study of a strange bleeding disorder that suddenly occurred in cattle on the northern prairies of the United States in the early 1900s.
The familiar sounds of your heart are made by the heart's valves as they open and close.
Cocaine was isolated in 1860 and first used as a local anesthetic in 1884. Its first clinical use was by Sigmund Freud to wean a patient from morphine addiction. The fictional character Sherlock Holmes was supposed to be addicted to cocaine by injection.