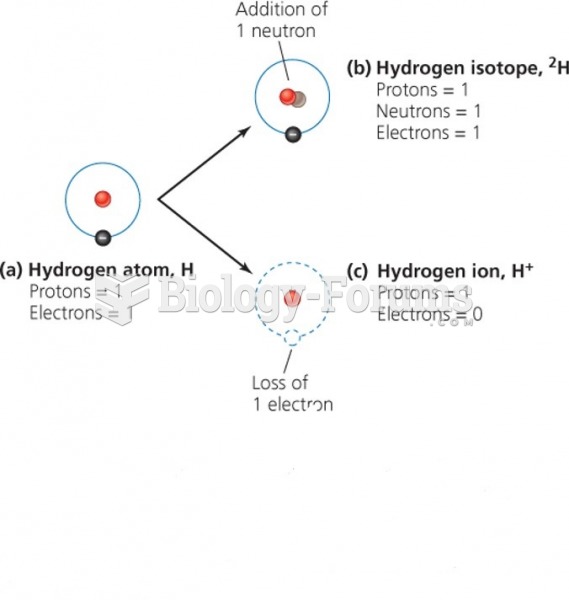
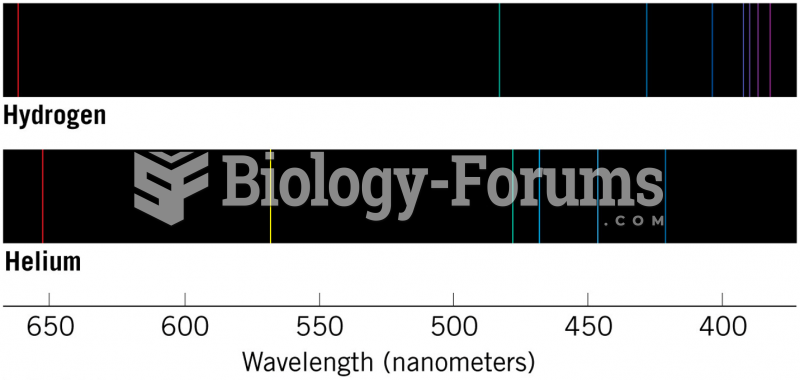
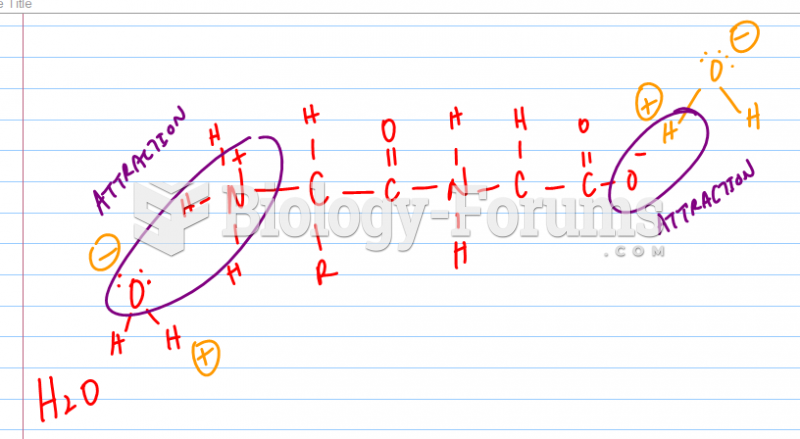
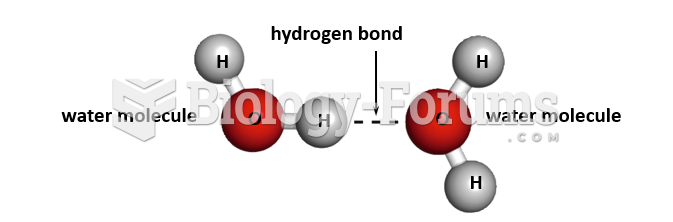
|
|
|
To combat osteoporosis, changes in lifestyle and diet are recommended. At-risk patients should include 1,200 to 1,500 mg of calcium daily either via dietary means or with supplements.
Eat fiber! A diet high in fiber can help lower cholesterol levels by as much as 10%.
Stevens-Johnson syndrome and Toxic Epidermal Necrolysis syndrome are life-threatening reactions that can result in death. Complications include permanent blindness, dry-eye syndrome, lung damage, photophobia, asthma, chronic obstructive pulmonary disease, permanent loss of nail beds, scarring of mucous membranes, arthritis, and chronic fatigue syndrome. Many patients' pores scar shut, causing them to retain heat.
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
On average, the stomach produces 2 L of hydrochloric acid per day.