|
|
|
The first war in which wide-scale use of anesthetics occurred was the Civil War, and 80% of all wounds were in the extremities.
Less than one of every three adults with high LDL cholesterol has the condition under control. Only 48.1% with the condition are being treated for it.
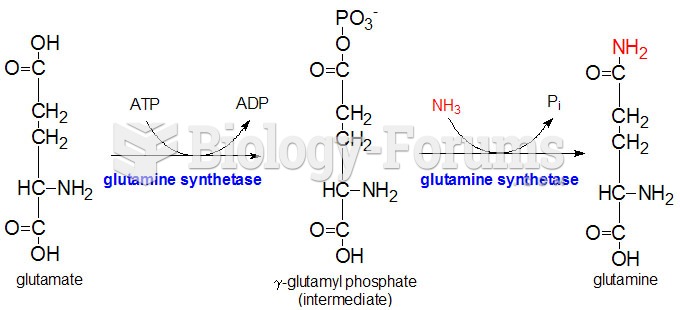
The use of salicylates dates back 2,500 years to Hippocrates's recommendation of willow bark (from which a salicylate is derived) as an aid to the pains of childbirth. However, overdosage of salicylates can harm body fluids, electrolytes, the CNS, the GI tract, the ears, the lungs, the blood, the liver, and the kidneys and cause coma or death.
Looking at the sun may not only cause headache and distort your vision temporarily, but it can also cause permanent eye damage. Any exposure to sunlight adds to the cumulative effects of ultraviolet (UV) radiation on your eyes. UV exposure has been linked to eye disorders such as macular degeneration, solar retinitis, and corneal dystrophies.
The average older adult in the United States takes five prescription drugs per day. Half of these drugs contain a sedative. Alcohol should therefore be avoided by most senior citizens because of the dangerous interactions between alcohol and sedatives.
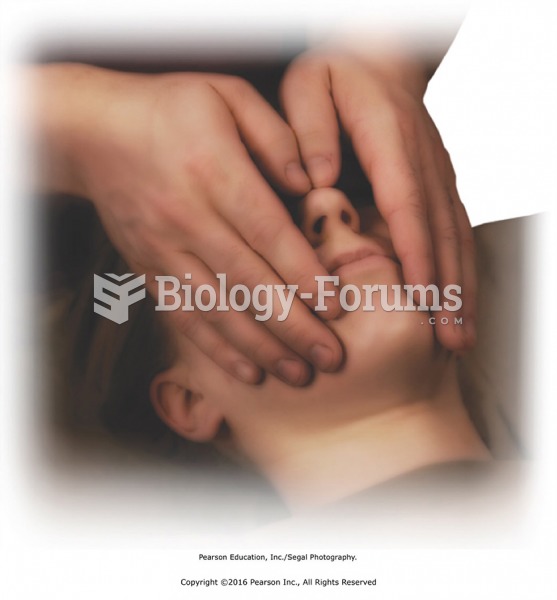
 The patient is undergoing an allergy skin test by receiving subdermal inoculations of allergens. Inf
The patient is undergoing an allergy skin test by receiving subdermal inoculations of allergens. Inf
 Correctly administering and interpreting an intelligence test is a skill that takes many years for ...
Correctly administering and interpreting an intelligence test is a skill that takes many years for ...