|
|
|
Amphetamine poisoning can cause intravascular coagulation, circulatory collapse, rhabdomyolysis, ischemic colitis, acute psychosis, hyperthermia, respiratory distress syndrome, and pericarditis.
The average adult has about 21 square feet of skin.
The liver is the only organ that has the ability to regenerate itself after certain types of damage. As much as 25% of the liver can be removed, and it will still regenerate back to its original shape and size. However, the liver cannot regenerate after severe damage caused by alcohol.
In 2012, nearly 24 milliion Americans, aged 12 and older, had abused an illicit drug, according to the National Institute on Drug Abuse (NIDA).
As many as 28% of hospitalized patients requiring mechanical ventilators to help them breathe (for more than 48 hours) will develop ventilator-associated pneumonia. Current therapy involves intravenous antibiotics, but new antibiotics that can be inhaled (and more directly treat the infection) are being developed.
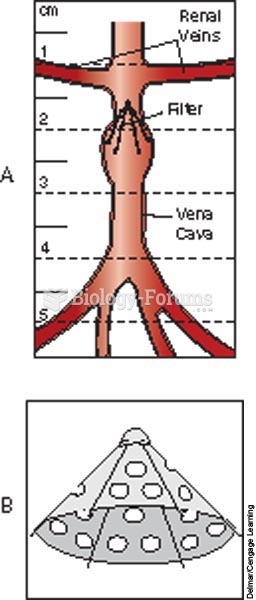 Filter in the vena cava prevents an embolus from traveling to the heart, lungs, or brain; A, Greenfi
Filter in the vena cava prevents an embolus from traveling to the heart, lungs, or brain; A, Greenfi
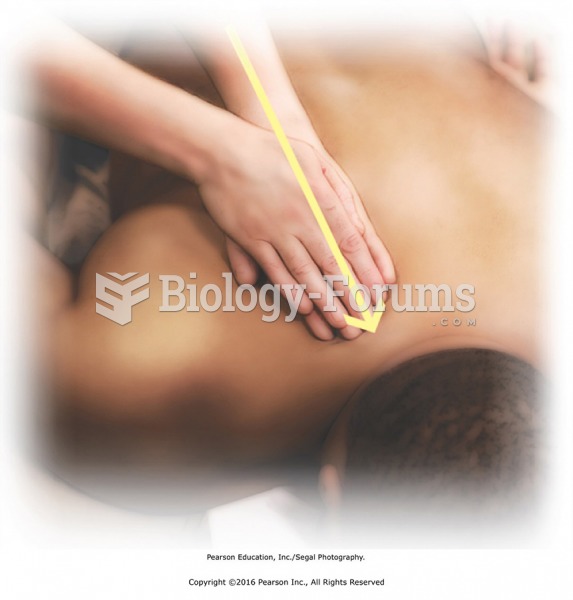 Reconnect the lower back with the shoulder with a few deep effleurage strokes. Then place one hand ...
Reconnect the lower back with the shoulder with a few deep effleurage strokes. Then place one hand ...