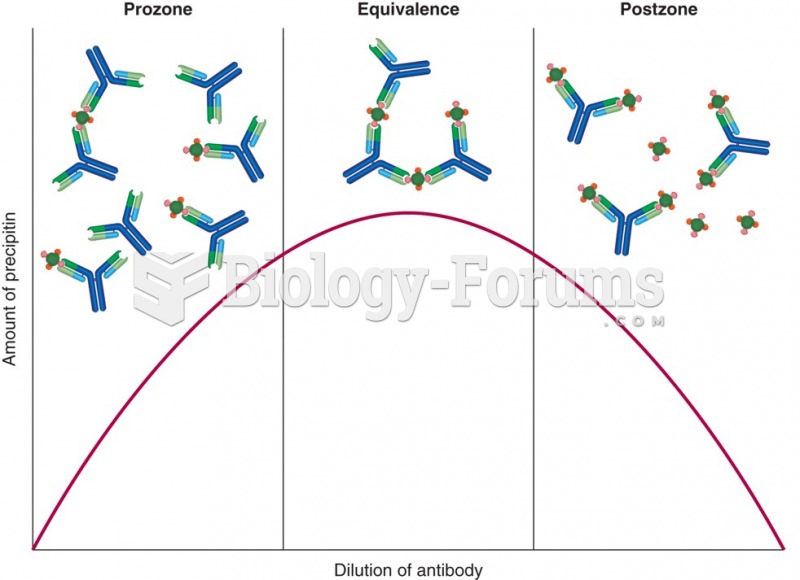
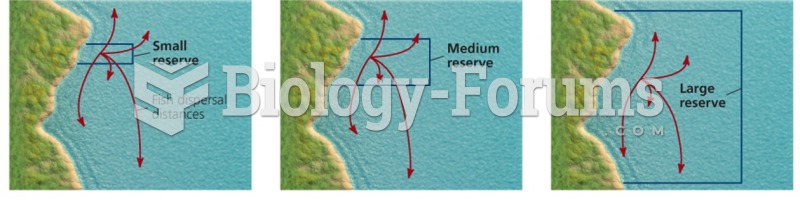
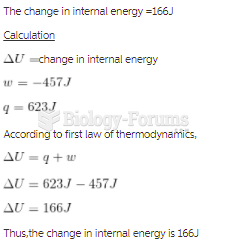This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Despite claims by manufacturers, the supplement known as Ginkgo biloba was shown in a study of more than 3,000 participants to be ineffective in reducing development of dementia and Alzheimer’s disease in older people.
Did you know?
Illicit drug use costs the United States approximately $181 billion every year.
Did you know?
Chronic marijuana use can damage the white blood cells and reduce the immune system's ability to respond to disease by as much as 40%. Without a strong immune system, the body is vulnerable to all kinds of degenerative and infectious diseases.
Did you know?
If all the neurons in the human body were lined up, they would stretch more than 600 miles.
Did you know?
There are more sensory neurons in the tongue than in any other part of the body.