This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Not getting enough sleep can greatly weaken the immune system. Lack of sleep makes you more likely to catch a cold, or more difficult to fight off an infection.
Did you know?
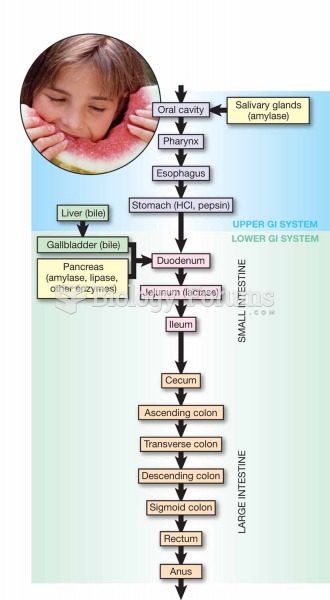
The horizontal fraction bar was introduced by the Arabs.
Did you know?
It is widely believed that giving a daily oral dose of aspirin to heart attack patients improves their chances of survival because the aspirin blocks the formation of new blood clots.
Did you know?
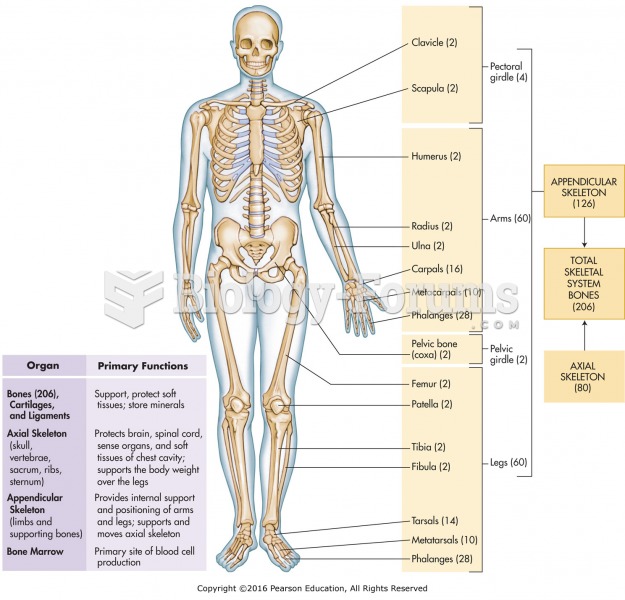
Congestive heart failure is a serious disorder that carries a reduced life expectancy. Heart failure is usually a chronic illness, and it may worsen with infection or other physical stressors.
Did you know?
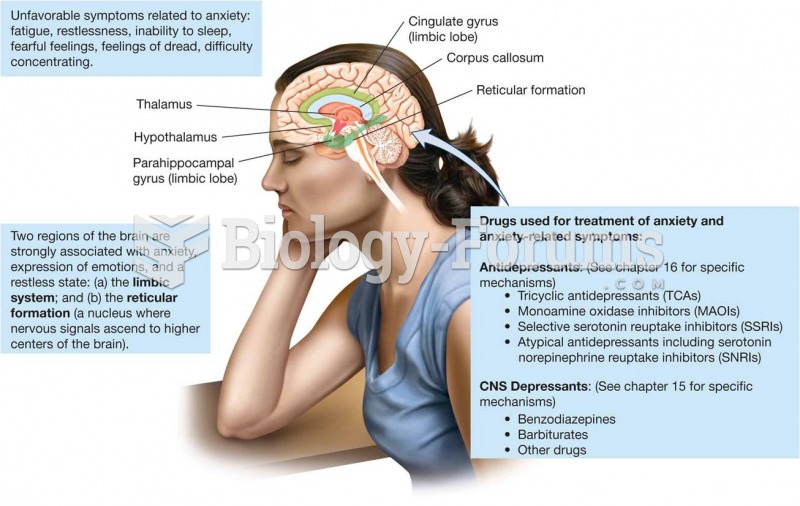
Individuals are never “cured” of addictions. Instead, they learn how to manage their disease to lead healthy, balanced lives.