|
|
|
According to the National Institute of Environmental Health Sciences, lung disease is the third leading killer in the United States, responsible for one in seven deaths. It is the leading cause of death among infants under the age of one year.
Approximately one in three babies in the United States is now delivered by cesarean section. The number of cesarean sections in the United States has risen 46% since 1996.
Street names for barbiturates include reds, red devils, yellow jackets, blue heavens, Christmas trees, and rainbows. They are commonly referred to as downers.
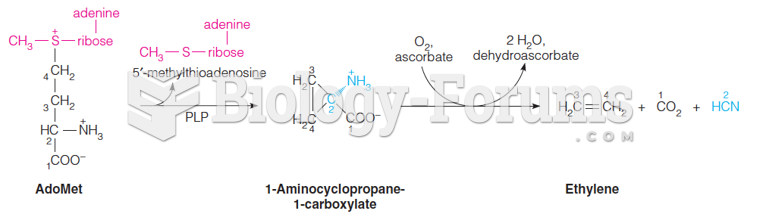
Certain rare plants containing cyanide include apricot pits and a type of potato called cassava. Fortunately, only chronic or massive ingestion of any of these plants can lead to serious poisoning.
The Romans did not use numerals to indicate fractions but instead used words to indicate parts of a whole.
 Used antifreeze coolant should be kept separate and stored in a leakproof container until it can be ...
Used antifreeze coolant should be kept separate and stored in a leakproof container until it can be ...
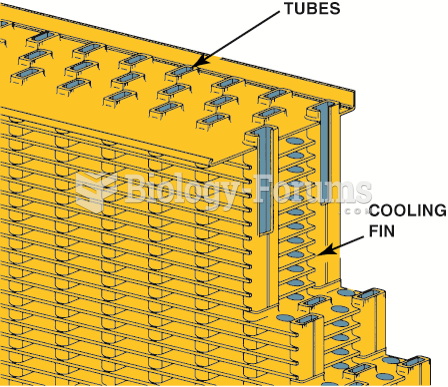
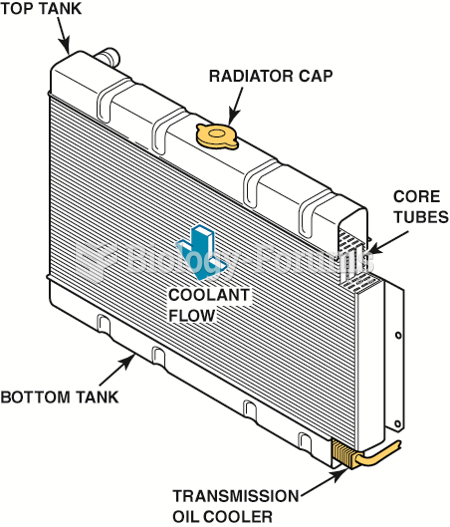
 A heavily corroded radiator from a vehicle that was overheating. A visual inspection discovered that ...
A heavily corroded radiator from a vehicle that was overheating. A visual inspection discovered that ...