This topic contains a solution. Click here to go to the answer
|
|
|
Did you know?
Blood is approximately twice as thick as water because of the cells and other components found in it.
Did you know?
Approximately 70% of expectant mothers report experiencing some symptoms of morning sickness during the first trimester of pregnancy.
Did you know?
Only one in 10 cancer deaths is caused by the primary tumor. The vast majority of cancer mortality is caused by cells breaking away from the main tumor and metastasizing to other parts of the body, such as the brain, bones, or liver.
Did you know?
Fungal nail infections account for up to 30% of all skin infections. They affect 5% of the general population—mostly people over the age of 70.
Did you know?
Blood in the urine can be a sign of a kidney stone, glomerulonephritis, or other kidney problems.
 Sunlight on Mars is dimmer than on Earth. This photo of a Martian sunset was imaged by Mars Pathfind
Sunlight on Mars is dimmer than on Earth. This photo of a Martian sunset was imaged by Mars Pathfind
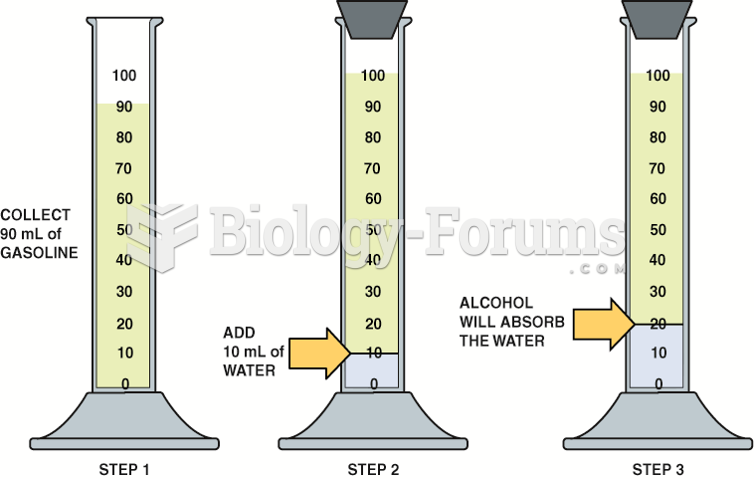 Checking gasoline for alcohol involves using a graduated cylinder and adding water to check if the ...
Checking gasoline for alcohol involves using a graduated cylinder and adding water to check if the ...